Bortezomib
These highlights do not include all the information needed to use BORTEZOMIB FOR INJECTION safely and effectively. See full prescribing information for BORTEZOMIB FOR INJECTION. BORTEZOMIB for injection, for subcutaneous or intravenous use Initial U.S. Approval: 2003
c6b620c6-f3d6-4ff3-b46a-50a66e6d41dd
HUMAN PRESCRIPTION DRUG LABEL
Jul 31, 2025
BluePoint Laboratories
DUNS: 985523874
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Bortezomib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Bortezomib -Carton Rev. 02/25
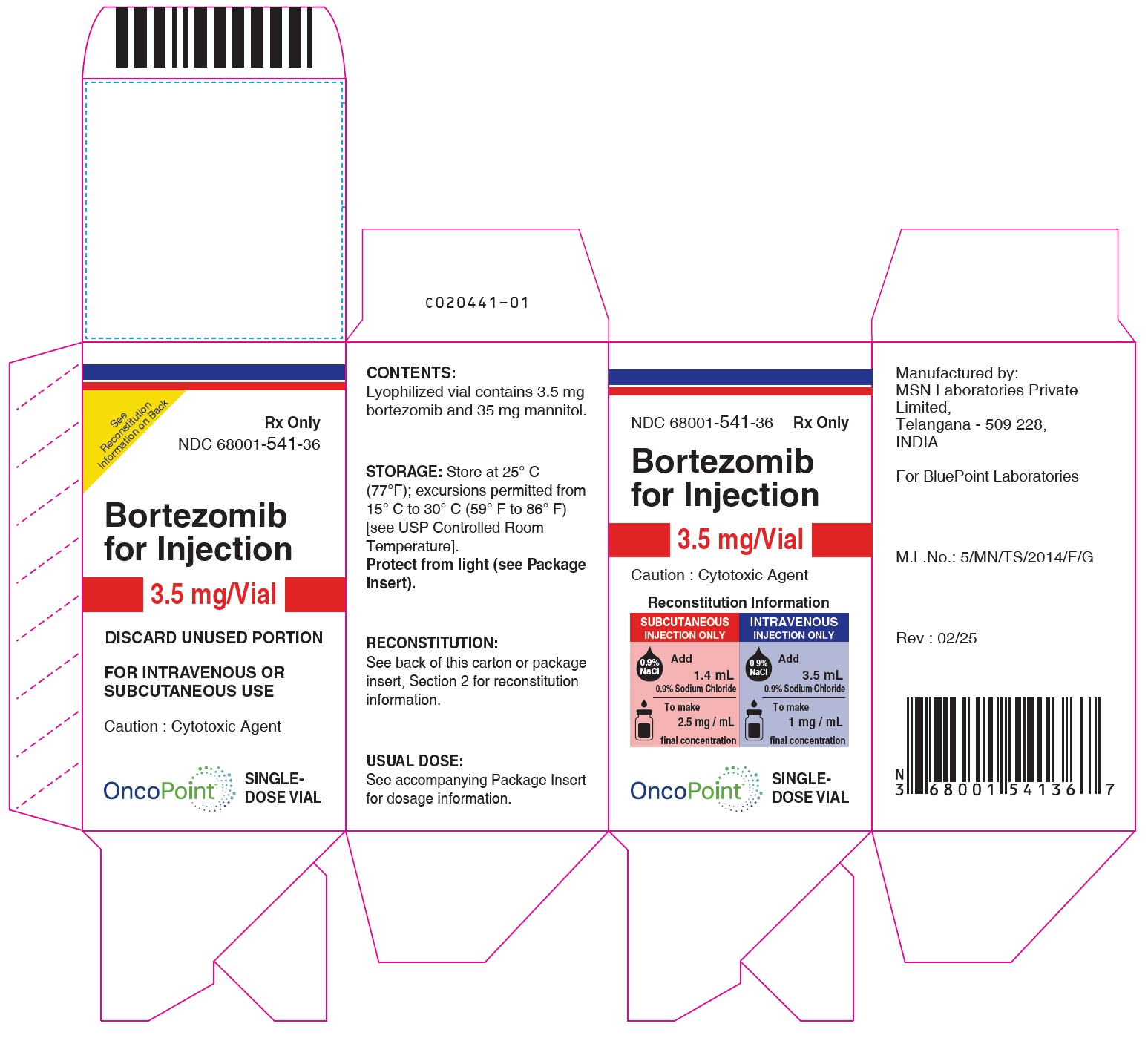
Bortezomib -Carton Rev. 05/22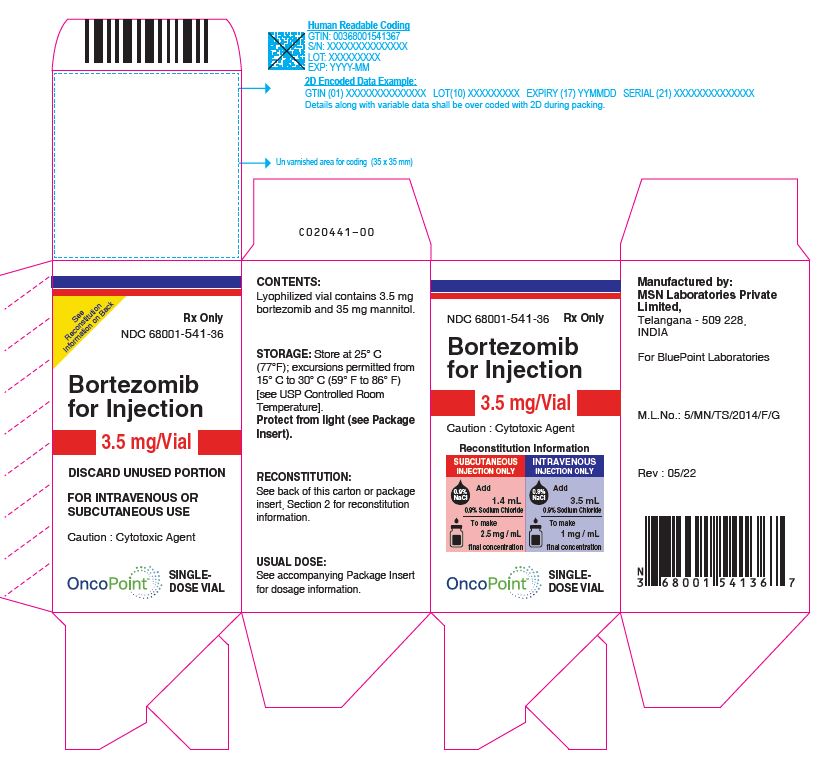
Bortezomib-Vial-Label Rev. 05/22
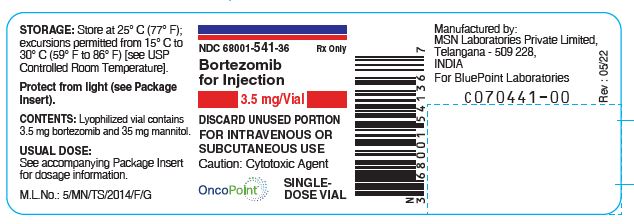
Bortezomib-intravenous-sticker-label

Bortezomib-subcutaneous-sticker-label

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
.
5.1 Peripheral Neuropathy
Bortezomib treatment causes a peripheral neuropathy that is predominantly sensory; however, cases of severe sensory and motor peripheral neuropathy have been reported. Patients with pre-existing symptoms (numbness, pain or a burning feeling in the feet or hands) and/or signs of peripheral neuropathy may experience worsening peripheral neuropathy (including ≥Grade 3) during treatment with bortezomib. Patients should be monitored for symptoms of neuropathy, such as a burning sensation, hyperesthesia, hypoesthesia, paresthesia, discomfort, neuropathic pain or weakness. In the Phase 3 relapsed multiple myeloma trial comparing bortezomib subcutaneous vs intravenous, the incidence of Grade ≥2 peripheral neuropathy was 24% for subcutaneous and 39% for intravenous. Grade ≥3 peripheral neuropathy occurred in 6% of patients in the subcutaneous treatment group, compared with 15% in the intravenous treatment group [see Adverse Reactions (6.1)]. Starting bortezomib subcutaneously may be considered for patients with pre-existing or at high risk of peripheral neuropathy.
Patients experiencing new or worsening peripheral neuropathy during bortezomib therapy may require a decrease in the dose and/or a less dose-intense schedule [see Dosage and Administration (2.7)]. In the bortezomib vs dexamethasone Phase 3 relapsed multiple myeloma study, improvement in or resolution of peripheral neuropathy was reported in 48% of patients with ≥Grade 2 peripheral neuropathy following dose adjustment or interruption. Improvement in or resolution of peripheral neuropathy was reported in 73% of patients who discontinued due to Grade 2 neuropathy or who had ≥Grade 3 peripheral neuropathy in the Phase 2 multiple myeloma studies. The long-term outcome of peripheral neuropathy has not been studied in mantle cell lymphoma.
5.2 Hypotension
The incidence of hypotension (postural, orthostatic, and hypotension NOS) was 8% [see Adverse Reactions (6.1)]. These events are observed throughout therapy. Patients with a history of syncope, patients receiving medications known to be associated with hypotension, and patients who are dehydrated may be at increased risk of hypotension. Management of orthostatic/postural hypotension may include adjustment of antihypertensive medications, hydration, and administration of mineralocorticoids and/or sympathomimetics.
5.3 Cardiac Toxicity
Acute development or exacerbation of congestive heart failure and new onset of decreased left ventricular ejection fraction have occurred during bortezomib therapy, including reports in patients with no risk factors for decreased left ventricular ejection fraction [see Adverse Reactions (6.1)]. Patients with risk factors for, or existing heart disease should be frequently monitored. In the relapsed multiple myeloma study of bortezomib vs dexamethasone, the incidence of any treatment-related cardiac disorder was 8% and 5% in the bortezomib and dexamethasone groups, respectively. The incidence of adverse reactions suggestive of heart failure (acute pulmonary edema, pulmonary edema, cardiac failure, congestive cardiac failure, cardiogenic shock) was ≤ 1% for each individual reaction in the bortezomib group. In the dexamethasone group the incidence was ≤1% for cardiac failure and congestive cardiac failure; there were no reported reactions of acute pulmonary edema, pulmonary edema, or cardiogenic shock. There have been isolated cases of QT-interval prolongation in clinical studies; causality has not been established.
5.4 Pulmonary Toxicity
Acute Respiratory Distress Syndrome (ARDS) and acute diffuse infiltrative pulmonary disease of unknown etiology such as pneumonitis, interstitial pneumonia, lung infiltration have occurred in patients receiving bortezomib. Some of these events have been fatal.
In a clinical trial, the first two patients given high-dose cytarabine (2g/m 2 per day) by continuous infusion with daunorubicin and bortezomib for relapsed acute myelogenous leukemia died of ARDS early in the course of therapy.
There have been reports of pulmonary hypertension associated with bortezomib administration in the absence of left heart failure or significant pulmonary disease.
In the event of new or worsening cardiopulmonary symptoms, consider interrupting bortezomib until a prompt and comprehensive diagnostic evaluation is conducted.
5.5 Posterior Reversible Encephalopathy Syndrome (PRES)
Posterior Reversible Encephalopathy Syndrome (PRES; formerly termed Reversible Posterior Leukoencephalopathy Syndrome (RPLS)) has occurred in patients receiving bortezomib. PRES is a rare, reversible, neurological disorder which can present with seizure, hypertension, headache, lethargy, confusion, blindness, and other visual and neurological disturbances. Brain imaging, preferably MRI (Magnetic Resonance Imaging), is used to confirm the diagnosis. In patients developing PRES, discontinue bortezomib. The safety of reinitiating bortezomib therapy in patients previously experiencing PRES is not known.
5.6 Gastrointestinal Toxicity
Bortezomib treatment can cause nausea, diarrhea, constipation, and vomiting [see Adverse Reactions (6.1)] sometimes requiring use of antiemetic and antidiarrheal medications. Ileus can occur. Fluid and electrolyte replacement should be administered to prevent dehydration. Interrupt bortezomib for severe symptoms.
5.7 Thrombocytopenia/Neutropenia
Bortezomib is associated with thrombocytopenia and neutropenia that follow a
cyclical pattern with nadirs occurring following the last dose of each cycle
and typically recovering prior to initiation of the subsequent cycle. The
cyclical pattern of platelet and neutrophil decreases and recovery remain
consistent in the studies of multiple myeloma and mantle cell lymphoma, with
no evidence of cumulative thrombocytopenia or neutropenia in the treatment
regimens studied.
Monitor complete blood counts (CBC) frequently during treatment with
bortezomib. Measure platelet counts prior to each dose of bortezomib. Adjust
dose/schedule for thrombocytopenia [see Dosage and Administration (2.6)].
Gastrointestinal and intracerebral hemorrhage has occurred during
thrombocytopenia in association with bortezomib. Support with transfusions and
supportive care, according to published guidelines.
In the single agent, relapsed multiple myeloma study of bortezomib vs
dexamethasone, the mean platelet count nadir measured was approximately 40% of
baseline. The severity of thrombocytopenia related to pretreatment platelet
count is shown in Table 8. The incidence of bleeding (≥Grade 3) was 2% on the
bortezomib arm and was <1% in the dexamethasone arm.
** Table 8: Severity of Thrombocytopenia Related to Pretreatment Platelet Count in the Relapsed Multiple Myeloma Study of Bortezomib vs Dexamethasone**
|
Pretreatment Platelet Count* |
Number of Patients (N=331)****‡ |
Number (%) of Patients with Platelet Count <10,000/µL |
Number (%) of Patients with Platelet Count 10,000 to 25,000/µL |
|
≥ 75,000/µL |
309 |
8 (3%) |
36 (12%) |
|
≥ 50,000/µL to <75,000/ µL |
14 |
2 (14%) |
11 (79%) |
|
≥ 10,000/µL to <50,000/ µL |
7 |
1 (14%) |
5 (71%) |
- A baseline platelet count of 50,000/µL was required for study eligibility
‡Data were missing at baseline for one patient
In the combination study of bortezomib with rituximab, cyclophosphamide,
doxorubicin and prednisone (BR-CAP) in previously untreated mantle cell
lymphoma patients, the incidence of thrombocytopenia (≥Grade 4) was 32% vs 1%
for the rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
(R-CHOP) arm as shown in Table 12. The incidence of bleeding events (≥Grade 3)
was 1.7% in the BR-CAP arm (four patients) and was 1.2% in the R-CHOP arm
(three patients).
Platelet transfusions were given to 23% of the patients in the BR-CAP arm and
3% of the patients in the R-CHOP arm.
The incidence of neutropenia (≥Grade 4) was 70% in the BR-CAP arm and was 52%
in the R-CHOP arm. The incidence of febrile neutropenia (≥Grade 4) was 5% in
the BR-CAP arm and was 6% in the R-CHOP arm. Myeloid growth factor support was
provided at a rate of 78% in the BR-CAP arm and 61% in the R-CHOP arm.
5.8 Tumor Lysis Syndrome
Tumor lysis syndrome has been reported with bortezomib therapy. Patients at risk of tumor lysis syndrome are those with high tumor burden prior to treatment. Monitor patients closely and take appropriate precautions.
5.9 Hepatic Toxicity
Cases of acute liver failure have been reported in patients receiving multiple concomitant medications and with serious underlying medical conditions. Other reported hepatic reactions include hepatitis, increases in liver enzymes, and hyperbilirubinemia. Interrupt bortezomib therapy to assess reversibility. There is limited rechallenge information in these patients.
5.10 Thrombotic Microangiopathy
Cases, sometimes fatal, of thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), have been reported in the postmarketing setting in patients who received bortezomib. Monitor for signs and symptoms of TTP/HUS. If the diagnosis is suspected, stop bortezomib and evaluate. If the diagnosis of TTP/HUS is excluded, consider restarting bortezomib. The safety of reinitiating bortezomib therapy in patients previously experiencing TTP/HUS is not known.
5.11 Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, bortezomib can cause
fetal harm when administered to a pregnant woman. Bortezomib administered to
rabbits during organogenesis at a dose approximately 0.5 times the clinical
dose of 1.3 mg/m 2 based on body surface area caused postimplantation loss and
a decreased number of live fetuses [ see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during
treatment with bortezomib and for seven months following treatment. Advise
males with female partners of reproductive potential to use effective
contraception during treatment with bortezomib and for four months following
treatment. If bortezomib is used during pregnancy or if the patient becomes
pregnant during bortezomib treatment, the patient should be apprised of the
potential risk to the fetus [ see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
- Peripheral Neuropathy: Manage with dose modification or discontinuation. ( 2.7) Patients with pre-existing severe neuropathy should be treated with bortezomib only after careful risk-benefit assessment. ( 2.7, 5.1)
- Hypotension: Use caution when treating patients taking anti‑hypertensives, with a history of syncope, or with dehydration. ( 5.2)
- Cardiac Toxicity: Worsening of and development of cardiac failure has occurred. Closely monitor patients with existing heart disease or risk factors for heart disease. ( 5.3)
- Pulmonary Toxicity: Acute respiratory syndromes have occurred. Monitor closely for new or worsening symptoms and consider interrupting bortezomib therapy. ( 5.4)
- Posterior Reversible Encephalopathy Syndrome: Consider MRI imaging for onset of visual or neurological symptoms; discontinue bortezomib if suspected. ( 5.5)
- Gastrointestinal Toxicity: Nausea, diarrhea, constipation, and vomiting may require use of antiemetic and antidiarrheal medications or fluid replacement. (5.6)
- Thrombocytopenia or Neutropenia: Monitor complete blood counts regularly throughout treatment. ( 5.7)
- Tumor Lysis Syndrome: Closely monitor patients with high tumor burden. ( 5.8)
- Hepatic Toxicity: Monitor hepatic enzymes during treatment. Interrupt bortezomib therapy to assess reversibility. ( 5.9)
- Thrombotic Microangiopathy: Monitor for signs and symptoms. Discontinue bortezomib if suspected. ( 5.10)
- Embryo-Fetal Toxicity: Bortezomib can cause fetal harm. Advise females of reproductive potential and males with female partners of reproductive potential of the potential risk to a fetus and to use effective contraception. ( 5.11)
DESCRIPTION SECTION
11 DESCRIPTION
Bortezomib for Injection, a proteasome inhibitor, contains bortezomib which is an antineoplastic agent. Bortezomib is a modified dipeptidyl boronic acid. The chemical name for bortezomib, the monomeric boronic acid, is [(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl) amino]propyl]amino]butyl] boronic acid.
Bortezomib has the following chemical structure:
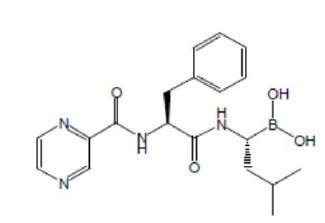
The molecular weight is 384.24. The molecular formula is C 19H 25BN 4O 4. The solubility of bortezomib, is freely soluble in N, N dimethyl fornamide, soluble in methanol and practically insoluble in n-Hexane.
Bortezomib is available for intravenous injection or subcutaneous use. Each single-dose vial contains 3.5 mg of bortezomib as a sterile lyophilized powder. It also contains the inactive ingredient: 35 mg mannitol, USP. The product is provided as a mannitol boronic ester which, in reconstituted form, consists of the mannitol ester in equilibrium with its hydrolysis product, the monomeric boronic acid. The drug substance exists in its cyclic anhydride form as a trimeric boroxine.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Multiple Myeloma
Randomized, Open-Label Clinical Study in Patients with Previously Untreated
Multiple Myeloma
A prospective, international, randomized (1:1), open-label clinical study
(NCT00111319) of 682 patients was conducted to determine whether bortezomib
administered intravenously (1.3 mg/m 2) in combination with melphalan (9 mg/m
2) and prednisone (60 mg/m 2) resulted in improvement in time to progression
(TTP) when compared to melphalan (9 mg/m 2) and prednisone (60 mg/m 2) in
patients with previously untreated multiple myeloma. Treatment was
administered for a maximum of nine cycles (approximately 54 weeks) and was
discontinued early for disease progression or unacceptable toxicity. Antiviral
prophylaxis was recommended for patients on the bortezomib study arm.
The median age of the patients in the study was 71 years (48;91), 50% were
male, 88% were Caucasian and the median Karnofsky performance status score for
the patients was 80 (60;100). Patients had IgG/IgA/Light chain myeloma in
63%/25%/8% instances, a median hemoglobin of 105 g/L (64;165), and a median
platelet count of 221,500/microliter (33,000; 587,000).
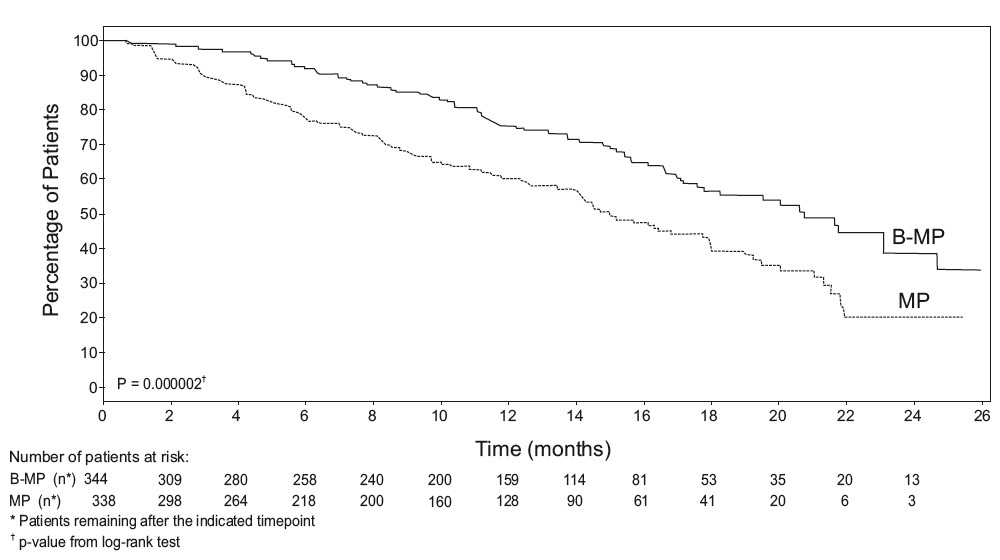
Efficacy results for the trial are presented in Table 14. At a prespecified
interim analysis (with median follow-up of 16.3 months), the combination of
bortezomib, melphalan and prednisone therapy resulted in significantly
superior results for time to progression, progression-free survival, overall
survival and response rate. Further enrollment was halted, and patients
receiving melphalan and prednisone were offered bortezomib in addition. A
later, prespecified analysis of overall survival (with median follow-up of
36.7 months with a hazard ratio of 0.65, 95% CI: 0.51, 0.84) resulted in a
statistically significant survival benefit for the bortezomib, melphalan and
prednisone treatment arm despite subsequent therapies including bortezomib
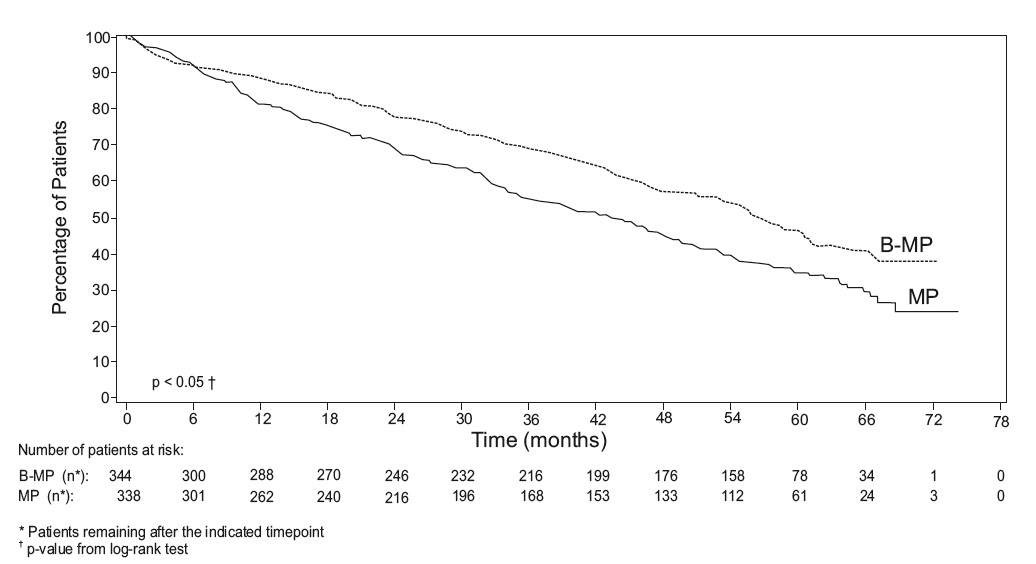
based regimens. In an updated analysis of overall survival based on 387 deaths
(median follow-up 60.1 months), the median overall survival for the
bortezomib, melphalan and prednisone treatment arm was 56.4 months and for the
melphalan and prednisone treatment arm was 43.1 months, with a hazard ratio of
0.695 (95% CI: 0.57, 0.85).
Table 14: Summary of Efficacy Analyses in the Previously Untreated Multiple Myeloma Study
|
Efficacy Endpoint |
Bortezomib, Melphalan and Prednisone |
Melphalan and Prednisone |
|
Time to Progression | ||
|
Events n (%) |
101 (29) |
152 (45) |
|
Median* (months) |
20.7 |
15.0 |
|
(95% CI) |
(17.6, 24.7) |
(14.1, 17.9) |
|
Hazard ratio † |
0.54 | |
|
(95% CI) |
(0.42, 0.70) | |
|
p-value ‡ |
0.000002 | |
|
Progression-Free Survival | ||
|
Events n (%) |
135 (39) |
190 (56) |
|
Median * (months) |
18.3 |
14.0 |
|
(95% CI) |
(16.6, 21.7) |
(11.1, 15.0) |
|
Hazard ratio † |
0.61 | |
|
(95% CI) |
(0.49, 0.76) | |
|
p-value ‡ |
0.00001 | |
|
Response Rate | ||
|
CR § n (%) |
102 (30) |
12 (4) |
|
PR § n (%) |
136 (40) |
103 (30) |
|
nCR n (%) |
5 (1) |
0 |
|
CR + PR § n (%) |
238 (69) |
115 (34) |
|
p-value ¶ |
<10 -10 | |
|
Overall Survival at Median Follow Up of 36.7 Months | ||
|
Events (deaths) n (%) |
109 (32) |
148 (44) |
|
Median * (months) |
Not Reached |
43.1 |
|
(95% CI) |
(46.2, NR) |
(34.8, NR) |
|
Hazard ratio † |
0.65 | |
|
(95% CI) |
(0.51, 0.84) | |
|
p-value ‡ |
0.00084 |
Note: All results are based on the analysis performed at a median follow-up
duration of 16.3 months except for the overall survival analysis.
*Kaplan-Meier estimate
†Hazard ratio estimate is based on a Cox proportional-hazard model adjusted
for stratification factors: beta 2-microglobulin, albumin, and region. A
hazard ratio less than one indicates an advantage for bortezomib, melphalan
and prednisone
‡p-value based on the stratified log-rank test adjusted for stratification
factors: beta 2-microglobulin, albumin, and region
§ EBMT criteria
¶ p-value for Response Rate (CR + PR) from the Cochran-Mantel-Haenszel chi-
square test adjusted for the stratification factors
TTP was statistically significantly longer on the bortezomib, melphalan and
prednisone arm (see Figure 1). (median follow-up 16.3 months)
Figure 1: Time to Progression Bortezomib, Melphalan and Prednisone vs
Melphalan and Prednisone
Overall survival was statistically significantly longer on the bortezomib,
melphalan and prednisone arm (see Figure 2). (median follow-up 60.1 months)
Figure 2: Overall Survival Bortezomib, Melphalan and Prednisone vs Melphalan
and Prednisone
Randomized, Clinical Study in Relapsed Multiple Myeloma of Bortezomib vs
Dexamethasone
A prospective Phase 3, international, randomized (1:1), stratified, open-label
clinical study (NCT00048230) enrolling 669 patients was designed to determine
whether bortezomib resulted in improvement in time to progression (TTP)
compared to high-dose dexamethasone in patients with progressive multiple
myeloma following 1 to 3 prior therapies. Patients considered to be refractory
to prior high-dose dexamethasone were excluded as were those with baseline
Grade ≥2 peripheral neuropathy or platelet counts < 50,000/μL. A total of 627
patients were evaluable for response.
Stratification factors were based on the number of lines of prior therapy the
patient had previously received (one previous line vs more than one line of
therapy), time of progression relative to prior treatment (progression during
or within six months of stopping their most recent therapy vs relapse > 6
months after receiving their most recent therapy), and screening beta
2-microglobulin levels (≤ 2.5 mg/L vs >2.5 mg/L).
Baseline patient and disease characteristics are summarized in Table 15.
Table 15: Summary of Baseline Patient and Disease Characteristics in the
Relapsed Multiple Myeloma Study
|
Patient Characteristics |
Bortezomib |
Dexamethasone |
|
Median age in years (range) |
62.0 (33, 84) |
61.0 (27, 86) |
|
Gender: Male/female |
56% / 44% |
60% / 40% |
|
Race: Caucasian/black/other |
90% / 6% / 4% |
88% / 7% / 5% |
|
Karnofsky performance status score ≤70 |
13% |
17% |
|
Hemoglobin <100 g/L |
32% |
28% |
|
Platelet count <75 x 10 9/L |
6% |
4% |
|
Disease Characteristics | ||
|
Type of myeloma (%): IgG/IgA/Light chain |
60% / 23% / 12% |
59% / 24% / 13% |
|
Median beta 2-microglobulin (mg/L) |
3.7 |
3.6 |
|
Median albumin (g/L) |
39.0 |
39.0 |
|
Creatinine clearance ≤30 mL/min [n (%)] |
17 (5%) |
11 (3%) |
|
Median Duration of Multiple Myeloma Since Diagnosis (Years) |
3.5 |
3.1 |
|
Number of Prior Therapeutic Lines of Treatment | ||
|
Median |
2 |
2 |
|
1 prior line |
40% |
35% |
|
60% |
65% |
|
Previous Therapy | ||
|
Any prior steroids, e.g., dexamethasone, VAD |
98% |
99% |
|
Any prior anthracyclines, e.g., VAD, mitoxantrone |
77% |
76% |
|
Any prior alkylating agents, e.g., MP, VBMCP |
91% |
92% |
|
Any prior thalidomide therapy |
48% |
50% |
|
Vinca alkaloids |
74% |
72% |
|
Prior stem cell transplant/other high-dose therapy |
67% |
68% |
|
Prior experimental or other types of therapy |
3% |
2% |
Patients in the bortezomib treatment group were to receive 8, three week
treatment cycles followed by 3, five week treatment cycles of bortezomib.
Patients achieving a CR were treated for four cycles beyond first evidence of
CR. Within each three week treatment cycle, bortezomib 1.3 mg/m 2/dose alone
was administered by intravenous bolus twice weekly for two weeks on Days 1, 4,
8, and 11 followed by a ten day rest period (Days 12 to 21). Within each five
week treatment cycle, bortezomib 1.3 mg/m 2/dose alone was administered by
intravenous bolus once weekly for four weeks on Days 1, 8, 15, and 22 followed
by a 13 day rest period (Days 23 to 35) [ see Dosage and Administration (2.2)].
Patients in the dexamethasone treatment group were to receive 4, five week
treatment cycles followed by 5, four week treatment cycles. Within each five
week treatment cycle, dexamethasone 40 mg/day PO was administered once daily
on Days 1 to 4, 9 to 12, and 17 to 20 followed by a 15 day rest period (Days
21 to 35). Within each four week treatment cycle, dexamethasone 40 mg/day PO
was administered once daily on Days 1 to 4 followed by a 24 day rest period
(Days 5 to 28). Patients with documented progressive disease on dexamethasone
were offered bortezomib at a standard dose and schedule on a companion study.
Following a preplanned interim analysis of time to progression, the
dexamethasone arm was halted and all patients randomized to dexamethasone were
offered bortezomib, regardless of disease status.
In the bortezomib arm, 34% of patients received at least one bortezomib dose
in all eight of the three week cycles of therapy, and 13% received at least
one dose in all 11 cycles. The average number of bortezomib doses during the
study was 22, with a range of 1 to 44. In the dexamethasone arm, 40% of
patients received at least one dose in all four of the five week treatment
cycles of therapy, and 6% received at least one dose in all nine cycles.
The time to event analyses and response rates from the relapsed multiple
myeloma study are presented in Table 16. Response and progression were
assessed using the European Group for Blood and Marrow Transplantation (EBMT)
criteria. Complete response (CR) required <5% plasma cells in the marrow, 100%
reduction in M-protein, and a negative immunofixation test (IF -). Partial
response (PR) requires ≥50% reduction in serum myeloma protein and ≥90%
reduction of urine myeloma protein on at least two occasions for a minimum of
at least six weeks along with stable bone disease and normal calcium. Near
complete response (nCR) was defined as meeting all the criteria for complete
response including 100% reduction in M-protein by protein electrophoresis;
however, M-protein was still detectable by immunofixation (IF +).
Table 16: Summary of Efficacy Analyses in the Relapsed Multiple Myeloma
Study
|
** Efficacy Endpoint** |
All Patients |
1 Prior Line of Therapy |
> 1 Prior Line of Therapy | |||
|
Bortezomib |
Dex |
Bortezomib |
Dex |
Bortezomib |
Dex | |
|
(n=333) |
(n=336) |
(n=132) |
(n=119) |
(n=200) |
(n=217) | |
|
Time to ProgressionEvents n (%) |
147 (44) |
196 (58) |
55 (42) |
64 (54) |
92 (46) |
132 (61) |
|
Median * |
6.2 mo |
3.5 mo |
7.0 mo |
5.6 mo (3.4, 6.3) |
4.9 mo |
2.9 mo (2.8, 3.5) |
|
Hazard ratio † |
0.55 |
0.55 (0.38, 0.81) |
0.54 (0.41, 0.72) | |||
|
p-value ‡ |
<0.0001 |
0.0019 |
<0.0001 | |||
|
Overall Survival |
51 (15) |
84 (25) |
12 (9) |
24 (20) |
39 (20) |
60 (28) |
|
Hazard ratio † |
0.57 |
0.39 |
0.65 | |||
|
p-value ‡, § |
<0.05 |
<0.05 |
<0.05 | |||
|
Response Rate |
n=315 |
n=312 |
n=128 |
n=110 |
n=187 |
n=202 |
|
CR #n (%) |
20 (6) |
2 (<1) |
8 (6) |
2 (2) |
12 (6) |
0 (0) |
|
PR #n(%) |
101 (32) |
54 (17) |
49 (38) |
27 (25) |
52 (28) |
27 (13) |
|
nCR #, ♠ n(%) |
21 (7) |
3 (<1) |
8 (6) |
2 (2) |
13 (7) |
1 (<1) |
|
CR + PR #n (%) |
121 (38) |
56 (18) |
57 (45) |
29 (26) |
64 (34) |
27 (13) |
|
p-value ♥ |
<0.0001 |
0.0035 |
<0.0001 |
- Kaplan-Meier estimate
† Hazard ratio is based on Cox proportional-hazard model with the treatment as single independent variable. A hazard ratio less than one indicates an advantage for bortezomib
‡ p-value based on the stratified log-rank test including randomization stratification factors
§ Precise p-value cannot be rendered
¶ Response population includes patients who had measurable disease at baseline and received at least one dose of study drug
EBMT criteria; nCR meets all EBMT criteria for CR but has positive IF. Under
EBMT criteria nCR is in the PR category
♠ In two patients, the IF was unknown
♥ p-value for Response Rate (CR + PR) from the Cochran-Mantel-Haenszel chi-
square test adjusted for the stratification factors
TTP was statistically significantly longer on the bortezomib arm (see Figure
3).
Figure 3: Time to Progression Bortezomib vs Dexamethasone (Relapsed Multiple
Myeloma Study)
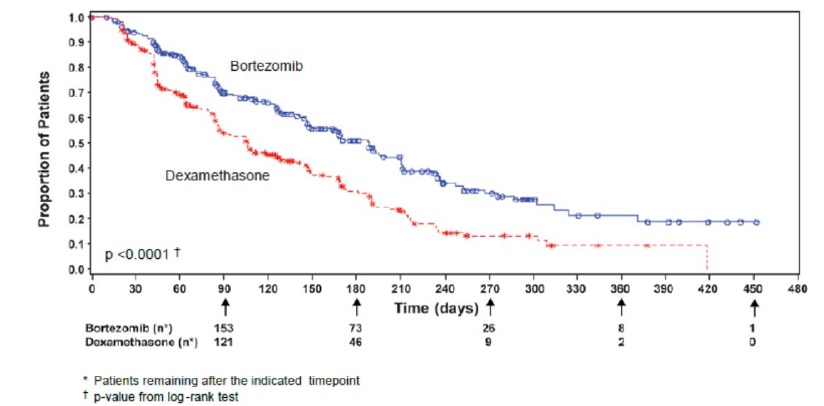
As shown in Figure 4, bortezomib had a significant survival advantage relative
to dexamethasone (p < 0.05). The median follow-up was 8.3 months.
Figure 4: Overall Survival Bortezomib vs Dexamethasone (Relapsed Multiple
Myeloma Study)
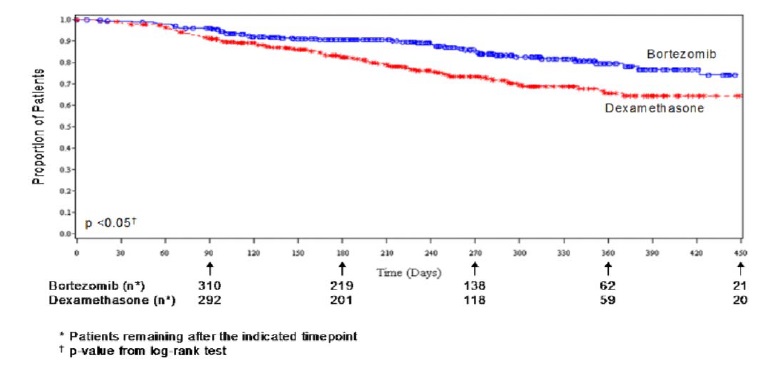
For the 121 patients achieving a response (CR or PR) on the bortezomib arm,
the median duration was 8.0 months (95% CI: 6.9, 11.5 months) compared to 5.6
months (95% CI: 4.8, 9.2 months) for the 56 responders on the dexamethasone
arm. The response rate was significantly higher on the bortezomib arm
regardless of beta 2-microglobulin levels at baseline.
Randomized, Open-Label Clinical Study of Bortezomib Subcutaneous vs
Intravenous in Relapsed Multiple Myeloma
An open-label, randomized, Phase 3 noninferiority study (NCT00722566) compared
the efficacy and safety of the subcutaneous administration of bortezomib vs
the intravenous administration. This study included 222 bortezomib naïve
patients with relapsed multiple myeloma, who were randomized in a 2:1 ratio to
receive 1.3 mg/m 2 of bortezomib by either the subcutaneous (n=148) or
intravenous (n=74) route for eight cycles. Patients who did not obtain an
optimal response (less than Complete Response (CR)) to therapy with bortezomib
alone after four cycles were allowed to receive oral dexamethasone 20 mg daily
on the day of and after bortezomib administration (82 patients in subcutaneous
treatment group and 39 patients in the intravenous treatment group). Patients
with baseline Grade ≥2 peripheral neuropathy or neuropathic pain, or platelet
counts <50,000/μL were excluded. A total of 218 patients were evaluable for
response.
Stratification factors were based on the number of lines of prior therapy the
patient had received ( one previous line vs more than one line of therapy),
and international staging system (ISS) stage (incorporating beta
2-microglobulin and albumin levels; Stages I, II, or III).
The baseline demographic and other characteristics of the two treatment groups
are summarized as follows: the median age of the patient population was
approximately 64 years of age (range: 38 to 88 years), primarily male
(subcutaneous: 50%, intravenous: 64%); the primary type of myeloma is IgG
(subcutaneous: 65% IgG, 26% IgA, 8% light chain; intravenous: 72% IgG, 19%
IgA, 8% light chain), ISS staging I/II/III (%) was 27, 41, 32 for both
subcutaneous and intravenous, Karnofsky performance status score was ≤70% in
22% of subcutaneous and 16% of intravenous, creatinine clearance was 67.5
mL/min in subcutaneous and 73 mL/min in intravenous, the median years from
diagnosis was 2.68 and 2.93 in subcutaneous and intravenous respectively and
the proportion of patients with more than one prior line of therapy was 38% in
subcutaneous and 35% in intravenous.
This study met its primary (noninferiority) objective that single agent
subcutaneous bortezomib retains at least 60% of the overall response rate
after four cycles relative to single agent intravenous bortezomib. The results
are provided in Table 17.
Table 17: Summary of Efficacy Analyses in the Relapsed Multiple Myeloma
Study of Bortezomib Subcutaneous vs Intravenous
|
Subcutaneous Bortezomib |
Intravenous Bortezomib | |
|
Intent to Treat Population |
(n=148) |
(n=74) |
|
Primary Endpoint | ||
|
Response Rate at 4 Cycles | ||
|
ORR (CR + PR) n(%) |
63 (43) |
31 (42) |
|
Ratio of Response Rates (95% CI) |
1.01 (0.73, 1.40) | |
|
CR n (%) |
11 (7) |
6 (8) |
|
PR n (%) |
52 (35) |
25 (34) |
|
nCR n (%) |
9 (6) |
4 (5) |
|
Secondary Endpoints | ||
|
Response Rate at 8 Cycles | ||
|
ORR (CR + PR) |
78 (53) |
38 (51) |
|
CR n (%) |
17 (11) |
9 (12) |
|
PR n (%) |
61 (41) |
29 (39) |
|
nCR n (%) |
14 (9) |
7 (9) |
|
Median Time to Progression, months |
10.4 |
9.4 |
|
Median Progression-Free Survival, months |
10.2 |
8.0 |
|
1 Year Overall Survival (%)***** |
72.6 |
76.7 |
*Median duration of follow-up is 11.8 months
A Randomized, Phase 2 Dose-Response Study in Relapsed Multiple Myeloma
An open-label, multicenter study randomized 54 patients with multiple myeloma
who had progressed or relapsed on or after front-line therapy to receive
bortezomib 1 mg/m 2 or 1.3 mg/m 2 intravenous bolus twice weekly for two weeks
on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21). The
median duration of time between diagnosis of multiple myeloma and first dose
of bortezomib on this trial was two years, and patients had received a median
of one prior line of treatment (median of three prior therapies). A single
complete response was seen at each dose. The overall response rates (CR + PR)
were 30% (8/27) at 1 mg/m 2 and 38% (10/26) at 1.3 mg/m 2.
A Phase 2 Open-Label Extension Study in Relapsed Multiple Myeloma
Patients from the two Phase 2 studies, who in the investigators’ opinion would
experience additional clinical benefit, continued to receive bortezomib beyond
8 cycles on an extension study. Sixty-three (63) patients from the Phase 2
multiple myeloma studies were enrolled and received a median of seven
additional cycles of bortezomib therapy for a total median of 14 cycles
(range: 7 to 32). The overall median dosing intensity was the same in both the
parent protocol and extension study. Sixty-seven percent (67%) of patients
initiated the extension study at the same or higher dose intensity at which
they completed the parent protocol, and 89% of patients maintained the
standard three week dosing schedule during the extension study. No new
cumulative or new long-term toxicities were observed with prolonged bortezomib
treatment [ see Adverse Reactions (6.1)].
A Single-Arm Trial of Retreatment in Relapsed Multiple Myeloma
A single-arm, open-label trial (NCT00431769) was conducted to determine the
efficacy and safety of retreatment with bortezomib. One hundred and thirty
patients (≥ 18 years of age) with multiple myeloma who previously had at least
partial response on a bortezomib-containing regimen (median of two prior lines
of therapy [range: 1to 7]) were retreated upon progression with bortezomib
administered intravenously. Patients were excluded from trial participation if
they had peripheral neuropathy or neuropathic pain of Grade ≥2. At least six
months after prior bortezomib therapy, bortezomib was restarted at the last
tolerated dose of 1.3 mg/m 2 (n=93) or ≤ 1 mg/m 2 (n=37) and given on Days 1,
4, 8 and 11 every three weeks for maximum of eight cycles either as single
agent or in combination with dexamethasone in accordance with the standard of
care. Dexamethasone was administered in combination with bortezomib to 83
patients in Cycle 1 with an additional 11 patients receiving dexamethasone
during the course of bortezomib retreatment cycles.
The primary endpoint was best confirmed response to retreatment as assessed by
European Group for Blood and Marrow Transplantation (EBMT) criteria. Fifty of
the 130 patients achieved a best confirmed response of Partial Response or
better for an overall response rate of 38.5% (95% CI: 30.1, 47.4). One patient
achieved a Complete Response and 49 achieved Partial Response. In the 50
responding patients, the median duration of response was 6.5 months and the
range was 0.6 to 19.3 months.
14.2 Mantle Cell Lymphoma
A Randomized, Open-Label Clinical Study in Patients with Previously Untreated
Mantle Cell Lymphoma
A randomized, open-label, Phase 3 study (NCT00722137) was conducted in 487
adult patients with previously untreated mantle cell lymphoma (Stage II, III
or IV) who were ineligible or not considered for bone marrow transplantation
to determine whether bortezomib administered in combination with rituximab,
cyclophosphamide, doxorubicin, and prednisone (BR-CAP) resulted in improvement
in progression-free survival (PFS) when compared to the combination of
rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
(R-CHOP). This clinical study utilized independent pathology confirmation and
independent radiologic response assessment.
Patients in the BR-CAP treatment arm received bortezomib (1.3 mg/m 2)
administered intravenously on Days 1, 4, 8, and 11 (rest period Days 12 to
21); rituximab (375 mg/m 2) on Day 1; cyclophosphamide (750 mg/m 2) on Day 1;
doxorubicin (50 mg/m 2) on Day 1; and prednisone (100 mg/m 2) on Day 1 through
Day 5 of the 21 day treatment cycle. For patients with a response first
documented at Cycle 6, two additional treatment cycles were allowed.
Median patient age was 66 years, 74% were male, 66% were Caucasian and 32%
were Asian. Sixty-nine percent of patients had a positive bone marrow aspirate
and/or a positive bone marrow biopsy for MCL, 54% of patients had an
International Prognostic Index (IPI) score of three (high‑intermediate) or
higher and 76% had Stage IV disease.
The majority of the patients in both groups received six or more cycles of
treatment, 84% in the BR-CAP group and 83% in the R-CHOP group. Median number
of cycles received by patients in both treatment arms was six with 17% of
patients in the R-CHOP group and 14% of subjects in the BR-CAP group receiving
up to two additional cycles.
The efficacy results for PFS, CR and ORR with a median follow-up of 40 months
are presented in Table 18. The response criteria used to assess efficacy were
based on the International Workshop to Standardize Response Criteria for Non-
Hodgkin’s Lymphoma (IWRC). Final overall survival results at a median follow-
up of 78.5 months are also presented in Table 18 and Figure 6. The combination
of BR-CAP resulted in statistically significant prolongation of PFS compared
with R-CHOP (see Table 18, Figure 5).
Table 18: Summary of Efficacy Analyses in the Previously Untreated Mantle
Cell Lymphoma Study
|
Efficacy Endpoint n: Intent to Treat patients |
BR-CAP |
R-CHOP |
|
Progression-Free Survival (by independent radiographic assessment) | ||
|
Events n (%) |
133 (55) |
165 (68) |
|
Median* (months) |
25 |
14 |
|
Hazard ratio † |
0.63 | |
|
p-value ‡ |
<0.001 | |
|
Complete Response Rate(CR) § | ||
|
n (%) |
108 (44) |
82 (34) |
|
(95% CI) |
(38, 51) |
(28, 40) |
|
Overall Response Rate (CR + Cru + PR)****¶ | ||
|
n (%) |
214 (88) |
208 (85) |
|
(95% CI) |
(83, 92) |
(80, 89) |
|
** Overall Survival** | ||
|
Events n(%) |
103 (42) |
138 (57) |
|
Median* (months) |
91 (71, NE) |
56 (47, 69) |
|
Hazard Ratio † |
0.66 |
Note: All results are based on the analysis performed at a median follow-up duration of 40 months except for the overall survival analysis, which was performed at a median follow-up of 78.5 months.
CI = Confidence Interval; IPI = International Prognostic Index; LDH = Lactate dehydrogenase
- Based on Kaplan-Meier product limit estimates.
† Hazard ratio estimate is based on a Cox’s model stratified by IPI risk and stage of disease. A hazard ratio <1 indicates an advantage for BR-CAP.
‡ Based on Log rank test stratified with IPI risk and stage of disease.
§ Includes CR by independent radiographic assessment, bone marrow, and LDH using ITT population.
¶ Includes CR + Cru + PR by independent radiographic assessment, regardless of the verification by bone marrow and LDH, using ITT population.
Figure 5: Progression-Free Survival BR-CAP vs R-CHOP (previously Untreated Mantle Cell Lymphoma Study)
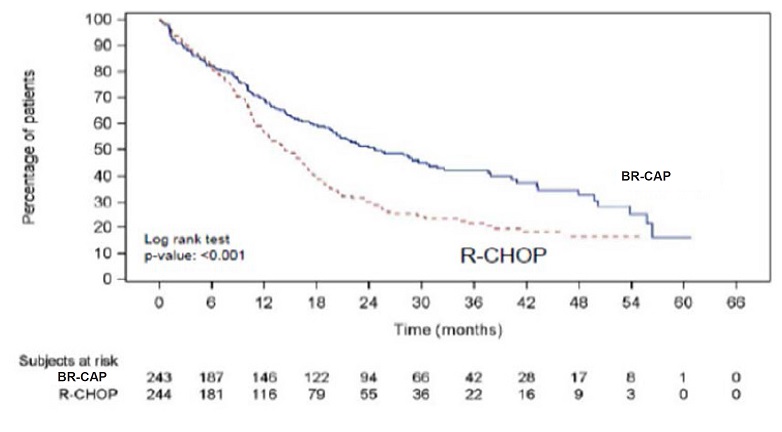
Key: R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone;
BR-CAP= bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone.
Figure 6: Overall Survival BR-CAP vs R-CHOP (previously Untreated Mantle Cell Lymphoma Study)
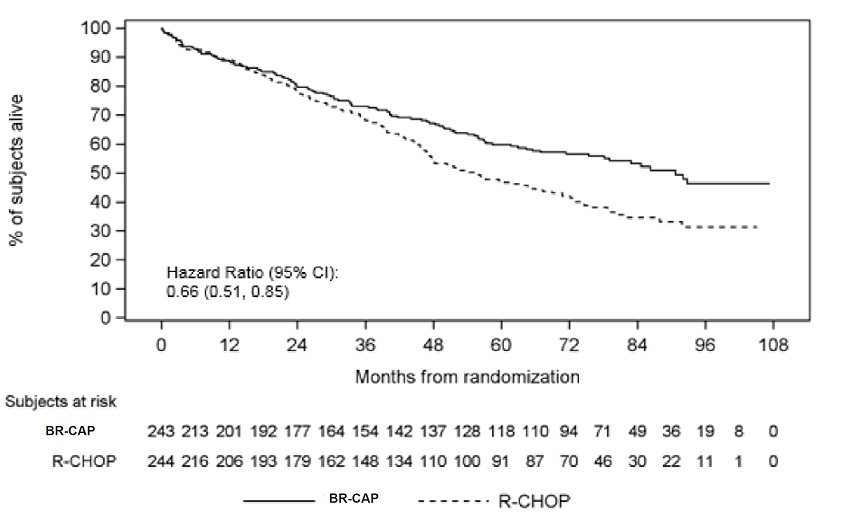
Key: R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and
prednisone;
BR-CAP = bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone.
A Phase 2 Single-Arm Clinical Study in Relapsed Mantle Cell Lymphoma after
Prior Therapy
The safety and efficacy of bortezomib in relapsed or refractory mantle cell
lymphoma were evaluated in an open-label, single-arm, multicenter study
(NCT00063713) of 155 patients with progressive disease who had received at
least one prior therapy. The median age of the patients was 65 years (42, 89),
81% were male, and 92% were Caucasian. Of the total, 75% had one or more
extra-nodal sites of disease, and 77% were Stage 4. In 91% of the patients,
prior therapy included all of the following: an anthracycline or mitoxantrone,
cyclophosphamide, and rituximab. A total of thirty-seven percent (37%) of
patients were refractory to their last prior therapy. An intravenous bolus
injection of bortezomib 1.3 mg/m 2/dose was administered twice weekly for two
weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to
21) for a maximum of 17 treatment cycles. Patients achieving a CR or CRu were
treated for four cycles beyond first evidence of CR or CRu. The study employed
dose modifications for toxicity [see Dosage and Administration (2.6, 2.7)].
Responses to bortezomib are shown in Table 19. Response rates to bortezomib
were determined according to the International Workshop Response Criteria
(IWRC) based on independent radiologic review of CT scans. The median number
of cycles administered across all patients was four; in responding patients
the median number of cycles was eight. The median time to response was 40 days
(range: 31 to 204 days). The median duration of follow-up was more than 13
months.
Table 19: Response Outcomes in a Phase 2 Relapsed Mantle Cell Lymphoma Study
|
Response Analyses (N = 155) |
N (%) |
95% CI |
|
Overall Response Rate (IWRC) (CR + CRu + PR) |
48 (31) |
(24, 39) |
|
Complete Response (CR + CRu) |
12 (8) |
(4, 13) |
|
CR |
10 (6) |
(3, 12) |
|
CRu |
2 (1) |
(0, 5) |
|
Partial Response (PR) |
36 (23) |
(17, 31) |
|
Duration of Response |
Median |
95% CI |
|
CR + CRu + PR (N = 48) |
9.3 months |
(5.4, 13.8) |
|
CR + CRu (N = 12) |
15.4 months |
(13.4, 15.4) |
|
PR (N=36) |
6.1 months |
(4.2, 9.3) |
