TRIHEXYPHENIDYL HYDROCHLORIDE
Novitium Pharma LLC. TRIHEXYPHENIDYL HYDROCHLORIDE Tablets, USP 2 mg and 5 mg Rx Only
7122d191-4972-4baf-a171-b7efd99d02ff
HUMAN PRESCRIPTION DRUG LABEL
Feb 6, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
TRIHEXYPHENIDYL HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Trihexyphenidyl hydrochloride Tablets, USP is a synthetic antispasmodic drug available in the following forms: Tablets, 2 mg and 5 mg. It is designated chemically 1-Piperidinepropanol,α-cyclohexyl-α-phenyl-, hydrochloride. The structural formula is represented below:
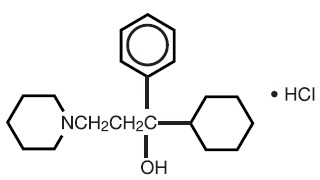
Trihexyphenidyl Hydrochloride Tablets, USP 2 mg and 5 mg contain the following inactive ingredients: Microcrystalline cellulose, magnesium stearate and sodium starch glycolate.
