EELHOE Skin Tag Patches
3ddd3fb0-b824-0cde-e063-6394a90aea6a
HUMAN OTC DRUG LABEL
Sep 2, 2025
Shantou Eelhoe Daily Chemical Technology Co., Ltd.
DUNS: 617290813
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GLYCERIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
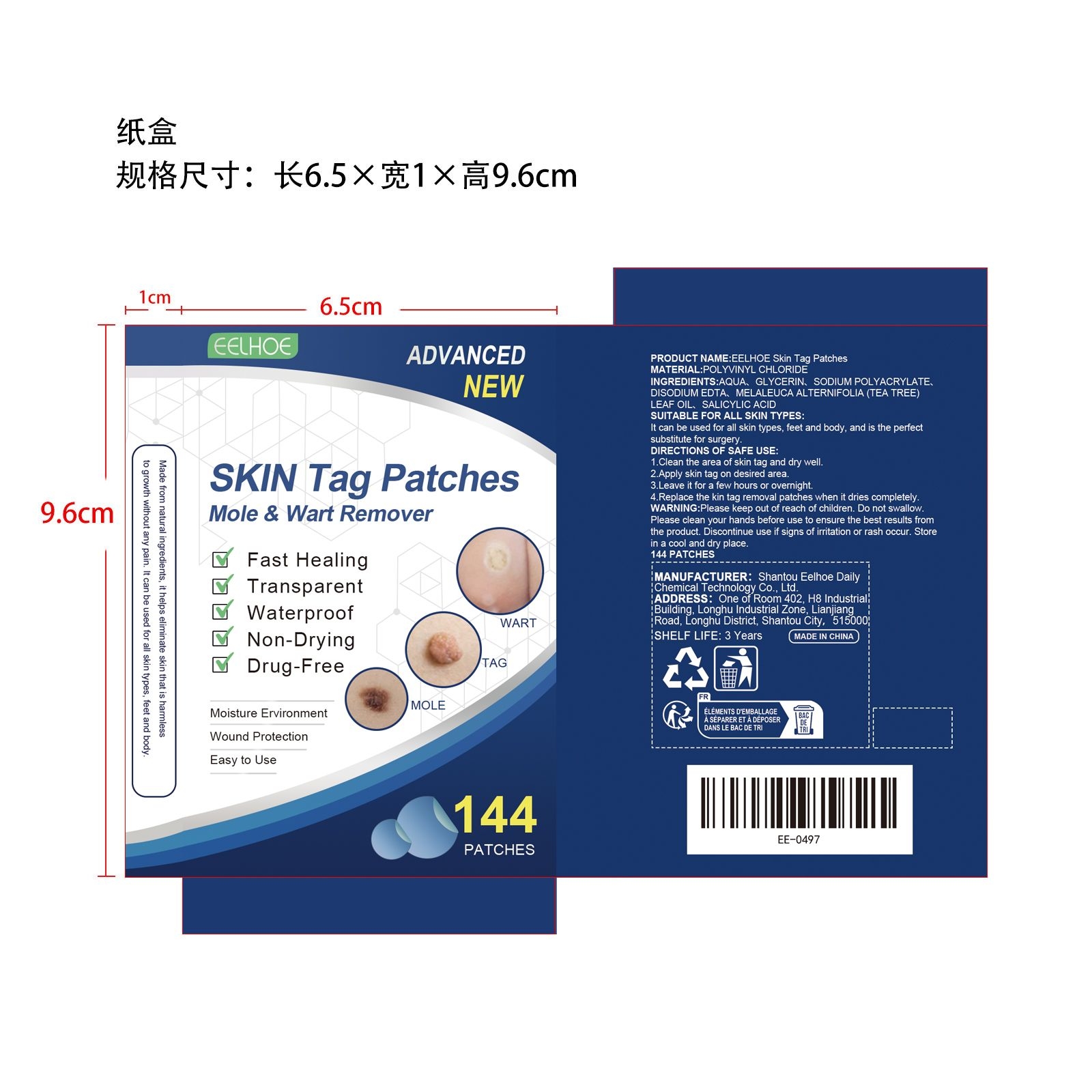
INDICATIONS & USAGE SECTION
1.Clean the area of skin tag and dry well.
2.Apply skin tag on desired area.
3.Leave it for a few hours or overnight.
4.Replace the kin tag removal patches when it dries completely.
STORAGE AND HANDLING SECTION
Store in a cool and dry place.
INACTIVE INGREDIENT SECTION
AQUA、GLYCERIN、SODIUM POLYACRYLATE、DISODIUM EDTA、SALICYLIC ACID
DOSAGE & ADMINISTRATION SECTION
1.Clean the area of skin tag and dry well.
2.Apply skin tag on desired area.
3.Leave it for a few hours or overnight.
4.Replace the kin tag removal patches when it dries completely.
OTC - ACTIVE INGREDIENT SECTION
MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL
OTC - WHEN USING SECTION
1.Clean the area of skin tag and dry well.
2.Apply skin tag on desired area.
3.Leave it for a few hours or overnight.
4.Replace the kin tag removal patches when it dries completely.
OTC - PURPOSE SECTION
Fast Healing
Transparent
Waterproof
Non-Drying
Drug-Free
WARNINGS SECTION
Please keep out of reach of children. Do not swallow.Please clean your hands before use to ensure the best results from the product. Discontinue use if signs of irritation or rash occur. Store in a cool and dry place.
OTC - DO NOT USE SECTION
Discontinue use if signs of irritation or rash occur.
OTC - STOP USE SECTION
Discontinue use if signs of irritation or rash occur.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Please keep out of reach of children. Do not swallow.
