Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Posology METALYSE should be administered as early as possible after symptom onset on the basis of body weight, with a maximum dose of 10,000 units (50 mg tenecteplase). The volume required to administer the correct dose can be calculated from the following scheme: 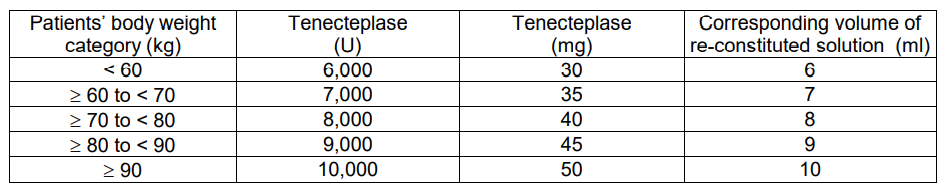 **Adjunctive therapy:** Antithrombotic adjunctive therapy is recommended according to the current international guidelines for the management of patients with ST-elevation myocardial infarction. For coronary intervention please refer to section 4.4 Special warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Method of administration The reconstituted solution should be administered intravenously and is for immediate use. The required dose should be administered as a single intravenous bolus over 5 to 10 seconds. **HANDLING INSTRUCTIONS** METALYSE should be reconstituted by adding the complete volume of water for injections from the pre-filled syringe to the vial containing the powder for injection. 01. Ensure that the appropriate vial size is chosen according to the body weight of the patient. (see section 4.2 Dosage and Administration) 02. Check that the cap of the vial is still intact. 03. Remove the flip-off cap from the vial. 04. Remove the tip-cap from the syringe. Then immediately screw the pre-filled syringe on the vial adapter and penetrate the vial stopper in the middle with the spike of the vial adapter. 05. Add the water for injections into the vial by pushing the syringe plunger down slowly to avoid foaming. 06. Keep the syringe attached to the vial adapter and reconstitute by swirling gently. 07. The reconstituted preparation is a colourless to pale yellow, clear solution. Only clear solution without particles should be used. 08. Directly before the solution is administered, invert the vial with the syringe still attached, so that the syringe is below the vial. 09. Transfer the appropriate volume of reconstituted solution of METALYSE into the syringe, based on the patient’s weight. 10. Unscrew the syringe from the vial adapter. 11. A pre-existing intravenous line, which has been used for administration of 0.9% sodium chloride solution only, may be used for administration of METALYSE. METALYSE should not be mixed with other drugs, neither in the same infusion-vial nor the same venous line (not even with heparin). 12. METALYSE should be administered to the patient, intravenously over 5 to 10 seconds. It should not be administered into a line containing dextrose as METALYSE is incompatible with dextrose solution. 13. The line should be flushed after METALYSE injection for proper delivery. 14. Any unused solution should be discarded. 15. Alternatively the reconstitution can be performed with a needle instead of the included vial adapter.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** METALYSE is indicated for the thrombolytic treatment of acute myocardial infarction (AMI) with persistent ST elevation or recent left Bundle Branch Block within 6 hours after the onset of acute myocardial infarction (AMI) symptoms.
**4.3 Contraindications** METALYSE is contraindicated in: - patients with known hypersensitivity to the active substance tenecteplase, gentamicin (a trace residue from the manufacturing process) or to any of the excipients - situations associated with a risk of bleeding such as: - Significant bleeding disorder at present or within the past 6 months, known haemorrhagic diathesis - Patients receiving effective oral anticoagulant treatment, e.g. warfarin sodium (INR > 1.3) (please see section 4.4 Special warnings and precautions, subsection “Bleeding” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - Any history of central nervous system damage (i.e. neoplasm, aneurysm, intracranial or spinal surgery) - Severe uncontrolled arterial hypertension - Major surgery, biopsy of a parenchymal organ, or significant trauma within the past 2 months (this includes any trauma associated with the current AMI), recent trauma to the head or cranium - Prolonged or traumatic cardiopulmonary resuscitation (> 2 minutes) within the past 2 weeks - Severe hepatic dysfunction, including hepatic failure, cirrhosis, portal hypertension (oesophageal varices) and active hepatitis - Active peptic ulceration - Arterial aneurysm and known arterial/venous malformation - Neoplasm with increased bleeding risk - Acute pericarditis and/or subacute bacterial endocarditis - Acute pancreatitis - Haemorrhagic stroke or stroke of unknown origin at any time. - Ischaemic stroke or transient ischaemic attack (TIA) in the preceding 6 months.
B01AD11
tenecteplase
Manufacturer Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
BOEHRINGER INGELHEIM PHARMA GMBH & CO KG
Active Ingredients
Documents
Package Inserts
Metalyse powder for injection PI.pdf
Approved: November 4, 2014
