Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2 DOSAGE AND ADMINISTRATION** **2.1 Patient Selection** Select patients for treatment of locally advanced or metastatic NSCLC with LUMAKRAS based on the presence of _KRAS G12C_ mutation in tumor or plasma specimens _\[see Clinical Studies (14)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. If no mutation is detected in a plasma specimen, test tumor tissue. **2.2 Recommended Dosage and Administration** The recommended dosage of LUMAKRAS is 960 mg (eight 120 mg tablets) orally once daily until disease progression or unacceptable toxicity. Take the daily dose of LUMAKRAS at the same time each day with or without food _\[see Clinical Pharmacology (12.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Swallow tablets whole. Do not chew, crush or split tablets. If a dose of LUMAKRAS is missed by more than 6 hours, take the next dose as prescribed the next day. Do not take 2 doses at the same time to make up for the missed dose. If vomiting occurs after taking LUMAKRAS, do not take an additional dose. Take the next dose as prescribed the next day. Administration to Patients Who Have Difficulty Swallowing Solids Disperse tablets in 120 mL (4 ounces) of non-carbonated, room-temperature water without crushing. No other liquids should be used. Stir or swirl the cup for approximately 3 minutes until tablets are dispersed into small pieces (the tablets will not completely dissolve) and drink immediately or within 2 hours. The appearance of the mixture may range from pale yellow to bright yellow. Swallow the tablet dispersion. Do not chew pieces of the tablet. Rinse the container with an additional 120 mL (4 ounces) of water and drink. If the mixture is not consumed immediately, stir the mixture again to ensure that tablets are dispersed. **2.3 Dosage Modifications for Adverse Reactions** LUMAKRAS dose reduction levels are summarized in Table 1. Dosage modifications for adverse reactions are provided in Table 2. If adverse reactions occur, a maximum of two dose reductions are permitted. Discontinue LUMAKRAS if patients are unable to tolerate the minimum dose of 240 mg once daily. 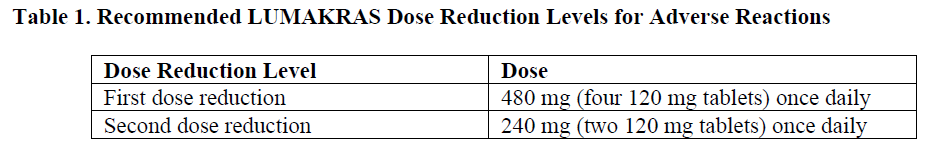 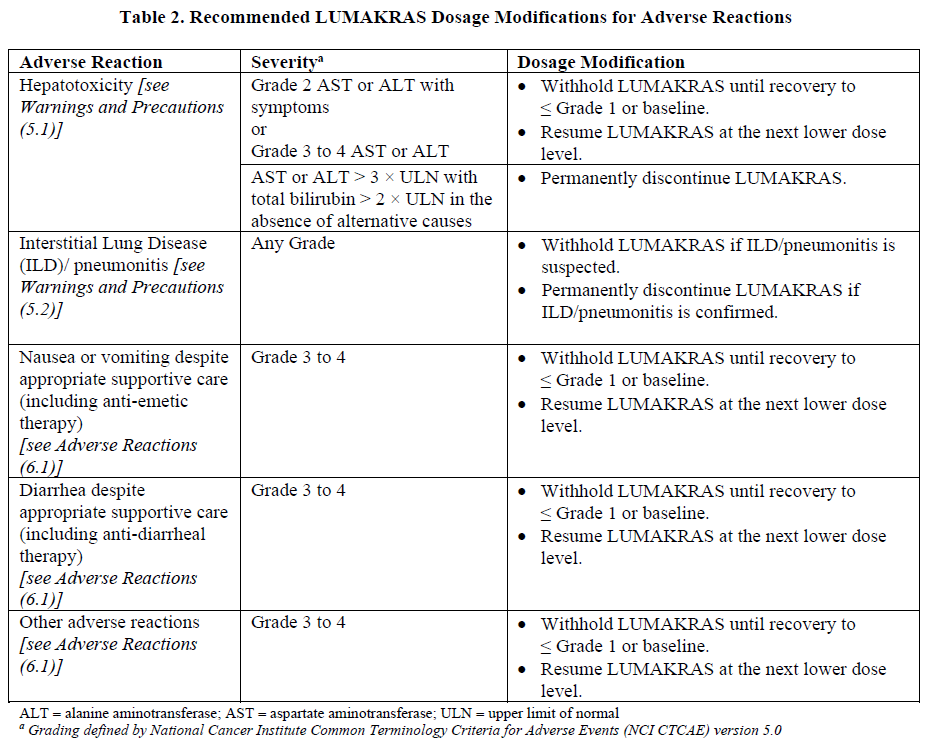 **2.4 Coadministration of LUMAKRAS with Acid-Reducing Agents** Avoid coadministration of proton pump inhibitors (PPIs) and H2 receptor antagonists with LUMAKRAS. If treatment with an acid-reducing agent cannot be avoided, take LUMAKRAS 4 hours before or 10 hours after administration of a local antacid _\[see Drug Interactions (7.1) and Clinical Pharmacology (12.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** LUMAKRAS is indicated for the treatment of adult patients with _KRAS G12C_-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), who have received at least one prior systemic therapy.
**4 CONTRAINDICATIONS** None.
L01XX73
sotorasib
Manufacturer Information
AMGEN BIOTECHNOLOGY SINGAPORE PTE. LTD.
Patheon Inc.
Active Ingredients
Documents
Package Inserts
Lumakras PI.pdf
Approved: June 28, 2022
