Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
PATCH
**Dosage and Administration** EVRA® should be applied to clean, dry, hairless, intact healthy skin on the buttock, abdomen, upper outer arm or upper torso, in a place where it will not be rubbed by tight clothing. EVRA® should not be placed on the breasts or on skin that is red, irritated or cut. To help avoid potential irritation, do not place a new patch on the same area of skin as the patch you have just removed, however, the patch may be applied within the same anatomic site. The patch should be pressed down firmly until the edges stick well. To prevent interference with the adhesive properties of EVRA®, no make-up, creams, lotions, powders or other topical products should be applied to the skin area where the EVRA® patch is currently placed or will be applied shortly. It is recommended that users visually check their patch daily to ensure continued proper adhesion. **Dosage** To achieve maximum contraceptive effectiveness, EVRA® must be used exactly as directed. Only one patch is to be worn at a time. The EVRA® patch should not be cut, damaged or altered in any way. If the EVRA® patch is cut, damaged or altered in size, contraceptive efficacy may be impaired. _**Adhesion of EVRA® patch**_ Patch adhesion was assessed indirectly by replacement rates for complete and partial patch detachment. Experience with more than 70,000 EVRA® patches worn for contraception for 6–13 cycles showed that 4.7% of patches were replaced because they either fell off (1.8%) or were partly detached (2.9%). Similarly, in a small study of patch wear under conditions of physical exertion and variable temperature and humidity, less than 2% of patches were replaced for complete or partial detachment. **Special populations** _**Pediatrics**_ Safety and efficacy of EVRA® was established in women from 18 years of age. Safety and efficacy are expected to be the same for post-pubertal adolescents and the same dosage is recommended in these subjects. Use of EVRA® before menarche is not indicated. _**Elderly**_ Not intended for use by post-menopausal women. _**Renal Impairment**_ EVRA® has not been studied in women with renal impairment. No dose adjustment is necessary but as there is a suggestion in the literature that the unbound fraction of EE is higher, EVRA® should be used with supervision in this population. _**Hepatic Impairment**_ EVRA® is contraindicated in this population. **Administration** To achieve maximum contraceptive effectiveness, EVRA® must be used exactly as directed. Complete instructions to facilitate patient counseling on proper system usage may be found in the Detailed Patient Labeling. _**Transdermal contraceptive system overview**_ This system uses a 28-day, four-week cycle. A new patch is applied each week for three weeks – 21 total days. Week Four is patch-free. Withdrawal bleeding is expected during this time. This means that every new patch will be applied on the same day of the week. This day is known as the “Patch Change Day”. For example, if the first patch is applied on a Monday, all subsequent patches should be applied on a Monday. Only one patch should be worn at a time. The EVRA® patch should not be cut, damaged or altered in any way. If the EVRA® patch is cut, damaged or altered in size, contraceptive efficacy may be impaired. On the day after Week Four ends a new four-week cycle is started by applying a new patch. Under no circumstances should there be more than a 7-day patch-free interval between dosing cycles. Clinical trials demonstrated that subjects randomized to EVRA® were able to adhere to the weekly dosing regimen better than with daily dosing of oral contraceptives.  If the patient is starting EVRA® for the **first time,** she should **wait until the day she begins her menstrual period**. Either a First Day start or Sunday start may be utilized (see below). The day she applies her first patch will be Day 1. Her “Patch Change Day” will be on this day every week. 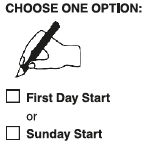 **First Day Start:** the patient should apply her first patch during the first 24 hours of her period. If therapy starts after Day 1 of the menstrual cycle, a non-hormonal contraceptive (such as a condom or diaphragm) should be used concurrently for the first 7 consecutive days of the first treatment cycle. OR **Sunday Start:** the patient should apply her first patch on the first Sunday after her period starts. She must use back-up contraception for the first week of her first cycle only. If the menstrual period begins on a Sunday, the first patch should be applied on that day. No back-up contraception is needed.  **Where to apply the patch.** The patch should be applied to clean, dry, intact healthy skin on the buttock, abdomen, upper outer arm or upper torso, in a place where it won’t be rubbed by tight clothing. EVRA® should not be placed on skin that is red, irritated or cut, nor should it be placed on the breasts. To prevent interference with the adhesive properties of EVRA®, no make-up, creams, lotions, powders or other topical products should be applied to the skin area where the EVRA® patch is currently placed or will be applied shortly. 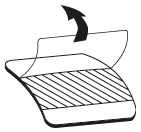 **Application of the EVRA® patch** The foil pouch is opened by tearing it along the edge using the fingers. A corner of the patch is grasped firmly and gently removed from the foil pouch. Sometimes patches can stick to the inside of the pouch – the patient should be careful not to accidentally remove the clear liner as she removes the patch. Then half of the clear protective liner is peeled away. The patient should avoid touching the sticky surface of the patch. 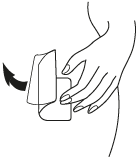 The patch is positioned on the skin and the other half of the liner is removed. The patient should press down firmly on the patch with the palm of her hand for 10 seconds, making sure that the edges stick well. She should check her patch every day to make sure it is sticking. 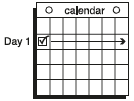 The patch is worn for 7 days (one week). On the “Patch Change Day”, Day 8, the used patch is removed and a new one is applied immediately. The used patch still contains some active hormones – it should be thrown away by carefully folding it in half so that it sticks to itself. 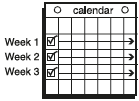 A new patch is applied on Week Two (Day 8) and again on Week Three (Day 15), on the usual “Patch Change Day”. Patch changes may occur at any time on the Change Day. Consecutive EVRA® patches should be applied to a new spot on the skin to help avoid potential irritation, although they may be kept within the same anatomic site. 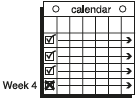 **Week Four is patch-free (Day 22 through Day 28),** thus completing the four-week contraceptive cycle. Bleeding is expected during this time.  **The next four-week cycle is started** by applying a new patch on the usual “Patch Change Day”, the day after Day 28, no matter when the menstrual period begins or ends. Under no circumstances should there be more than a 7-day patch free interval between dosing cycles. Patch adhesion was assessed indirectly by replacement rates for complete and partial patch detachment. Experience with more than 70,000 EVRA® patches worn for contraception for 6–13 cycles showed that 4.7% of patches were replaced because they either fell off (1.8%) or were partly detached (2.9%). Similarly, in a small study of patch wear under conditions of physical exertion and variable temperature and humidity, less than 2% of patches were replaced for complete or partial detachment. If the EVRA® patch becomes partially or completely detached and remains detached, insufficient drug delivery occurs. _If the patch remains even partly detached:_ - **for less than one day** (up to 24 hours), the patient should try to reapply it to the same place or replace it with a new patch immediately. No back-up contraception is needed. The woman’s “Patch Change Day” will remain the same. - **for more than one day (24 hours or more) OR if the patient is not sure how long the patch has been detached,** SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should stop the current contraceptive cycle and start a new cycle immediately by putting on a new patch. There is now a new “Day 1” and a new “Patch Change Day.” Back-up contraception must be used for the first week of the new cycle only. A patch should not be re-applied if it is no longer sticky, if it has become stuck to itself or another surface, if it has other material stuck to it or if it has become loose or fallen off before. If a patch cannot be re-attached, a new patch should be applied immediately. Supplemental adhesives or wraps should not be used to hold the EVRA® patch in place. **If the patient forgets to change her patch…** - **at the start of any patch cycle** (Week One/Day 1): SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should apply the first patch of her new cycle as soon as she remembers. There is now a new “Patch Change Day” and a new “Day 1”. The patient must use back-up contraception for the first week of her new cycle. - **in the middle of the patch cycle (Week Two/Day 8 or Week Three/Day 15),** - for **one or two days** (up to 48 hours), she should apply a new patch immediately. The next patch should be applied on the usual “Patch Change Day”. No back-up contraception is needed. - for **more than two days** (48 hours or more), SHE MAY NOT BE PROTECTED FROM PREGNANCY. She should stop the current contraceptive cycle and start a new four-week cycle immediately by putting on a new patch. There is now a new “Patch Change Day” and a new “Day 1”. The patient must use back-up contraception for one week. - **at the end of the patch cycle (Week Four/Day 22),** Week Four (Day 22): If the patient forgets to remove her patch, she should take it off as soon as she remembers. The next cycle should be started on the usual “Patch Change Day”, which is the day after Day 28. No back-up contraception is needed. **Under no circumstances should there be more than a 7-day patch-free interval between dosing cycles.** If there are more than 7 patch-free days, THE PATIENT MAY NOT BE PROTECTED FROM PREGNANCY and back-up contraception must be used concurrently for 7 days. As with combined oral contraceptives, the risk of ovulation increases with each day beyond the recommended contraceptive-free period. If coital exposure has occurred during such an extended patch-free interval, the possibility of fertilization should be considered. **Change Day Adjustment** If the patient wishes to move her Patch Change Day she should complete her current cycle, removing the third EVRA® patch on the correct day. During the patch-free week, a new Patch Change Day may be selected by applying a new EVRA® patch on the first occurrence of the desired day. In no case should there be more than 7 consecutive patch-free days. **Switching from an Oral Contraceptive** Treatment with EVRA® should begin on the first day of withdrawal bleeding. If there is no withdrawal bleeding within 5 days of the last active (hormone-containing) tablet, pregnancy must be ruled out prior to start of treatment with EVRA®. If therapy starts after the first day of withdrawal bleeding, a non-hormonal contraceptive must be used concurrently for 7 days. If more than 7 days elapse after taking the last active oral contraceptive tablet, the patient may have ovulated. The patient should be instructed to consult a physician before initiating treatment with EVRA®. If coital exposure has occurred during such an extended patch-free interval, the possibility of fertilization should be considered. **Use after Childbirth** Women who elect not to breastfeed should start contraceptive therapy with EVRA® no sooner than 4 weeks after childbirth. (see _Warnings and Precautions – Thromboembolic and other vascular disorders and Pregnancy and Breast-feeding_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) **Use after Abortion or Miscarriage** After an abortion or miscarriage that occurs before 20 weeks gestation, EVRA® may be started immediately. An additional method of contraception is not needed if EVRA® is started immediately. Be advised that ovulation may occur within 10 days of an abortion or miscarriage. After an abortion or miscarriage that occurs at or after 20 weeks gestation, EVRA® may be started either on Day 21 post-abortion or on the first day of the first spontaneous menstruation, whichever comes first. The incidence of ovulation on or before day 21 post-abortion (at 20 weeks gestation) is not known. **Breakthrough Bleeding or Spotting** In the event of breakthrough bleeding or spotting (bleeding that occurs during EVRA® usage), treatment should be continued. This type of bleeding usually disappears after the first few cycles. If breakthrough bleeding persists, a cause other than EVRA® should be considered. Two adequate and well-controlled trials demonstrated that the incidence of breakthrough bleeding and spotting with EVRA® is statistically and clinically comparable to that seen with ORTHO-CYCLEN® and TRIPHASIL®. In the event of no withdrawal bleeding (bleeding that should occur during the patch-free week), treatment should be continued on the next scheduled Change Day. If EVRA® has been used correctly, the absence of withdrawal bleeding is not necessarily an indication of pregnancy. Nevertheless, the possibility of pregnancy should be ruled out if absence of withdrawal bleeding occurs in 2 consecutive cycles. **In Case of Vomiting or Diarrhea** Unlike oral contraceptives, dose delivery by transdermal application should be unaffected by vomiting. Dose delivery is also expected to be unaffected by diarrhea. **In Case of Skin Irritation** If patch use results in uncomfortable irritation, a new patch may be applied to a new location until the next Change Day. Only one patch should be worn at a time. **Additional instructions for dosing** Breakthrough bleeding, spotting, and amenorrhea are frequent reasons for patients discontinuing hormonal contraceptives. In cases of breakthrough bleeding, structural abnormalities and dysfunctional uterine bleeding should be considered as potential causes. In undiagnosed persistent or recurrent abnormal bleeding from the vagina, adequate diagnostic measures are indicated to rule out pregnancy or malignancy. If pathology has been excluded, time or a change to another formulation may solve the problem. Changing to a hormonal contraceptive with a higher estrogen content, while potentially useful in minimizing menstrual irregularity, should be done only if necessary since this may increase the risk of thromboembolic disease. Use of hormonal contraceptives in the event of a missed menstrual period: 1. If the woman has not adhered to the prescribed schedule, the possibility of pregnancy should be considered at the time of the first missed period. Hormonal contraceptive use should be discontinued and a non-hormonal method should be used until pregnancy is ruled out. 2. If the woman has adhered to the prescribed regimen and misses one period, she should continue using her contraceptive patches. 3. If the woman has adhered to the prescribed regimen and misses two consecutive periods, pregnancy should be ruled out before continuing hormonal contraceptive use.
TRANSDERMAL
Medical Information
**Indications** Female Contraception
**Contraindications** EVRA® should not be used in women who currently have the following conditions: - Presence or risk of venous thromboembolism (VTE) - Venous thromboembolism – current VTE (on anticoagulants) or history of (e.g. deep venous thrombosis \[DVT\] or pulmonary embolism \[PE\]) - Known hereditary or acquired predisposition for venous thromboembolism, such as APC-resistance, (including Factor V Leiden), antithrombin-III-deficiency, protein C deficiency, protein S deficiency - Major surgery with prolonged immobilization (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - A high risk of venous thromboembolism due to the presence of multiple risk factors (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Presence or risk of arterial thromboembolism (ATE) - Arterial thromboembolism – current arterial thromboembolism, history of arterial thromboembolism (e.g. myocardial infarction) or prodromal condition (e.g. angina pectoris) - Cerebrovascular disease – current stroke, history of stroke or prodromal condition (e.g. transient ischaemic attack, TIA) - Known hereditary or acquired predisposition for arterial thromboembolism, such as hyperhomocysteinaemia and antiphospholipid-antibodies (anticardiolipin-antibodies, lupus anticoagulant). - History of migraine with focal neurological symptoms. - A high risk of arterial thromboembolism due to multiple risk factors (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) or to the presence of one serious risk factor such as: - Diabetes mellitus with vascular symptoms - Severe hypertension (Persistent blood pressure values of ≥ 160 mm Hg systolic or ≥ 100 mm Hg diastolic) - Severe dyslipoproteinaemia - Valvular heart disease with complications - Known or suspected carcinoma of the breast - Carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia - Undiagnosed abnormal genital bleeding - Cholestatic jaundice of pregnancy or jaundice with prior hormonal contraceptive use - Acute or chronic hepatocellular disease with abnormal liver function - Hepatic adenomas or carcinomas - Known or suspected pregnancy - Hypersensitivity to any component of this product - Patients receiving drug combinations with paritaprevir/ritonavir, ombitasvir, and/or dasabuvir due to potential for ALT elevations.
G03AA13
norelgestromin and ethinylestradiol
Manufacturer Information
ABBOTT LABORATORIES (SINGAPORE ) PRIVATE LIMITED
LTS LOHMANN THERAPIE-SYSTEME AG
Active Ingredients
Documents
Package Inserts
Evra_PI.pdf
Approved: June 20, 2023
