Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**DOSAGE AND ADMINISTRATION** _**General**_ Leuprorelin acetate for depot suspension must be administered under the supervision of a physician. As with other drugs administered by injection, the injection site should be varied periodically. Since the product does not contain a preservative, the suspension should be discarded if not used immediately. The recommended dose of leuprorelin acetate for depot suspension (11.25 mg) is 11.25 mg, administered as a single subcutaneous or intramuscular injection **every three months**. _**Prostate Cancer**_ In patients treated with GnRH analogues for prostate cancer, treatment is usually continued upon development of castration-resistant prostate cancer. Reference should be made to relevant guidelines. **_Preparation for Administration_** For optimal performance of the Prefilled Dual Chamber Syringe (PDS), read and follow the following instructions: 1. To prepare for injection, screw the white plunger into the end stopper until the stopper begins to turn. 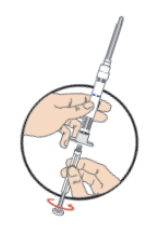 2. Hold the syringe UPRIGHT. Release the diluent by SLOWLY PUSHING (6 to 8 seconds) the plunger until the first stopper is at the blue line in the middle of the barrel. 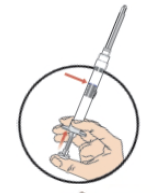 3. Keep the syringe UPRIGHT. Gently mix the microspheres (particles) thoroughly to form a uniform suspension. The suspension will appear milky. 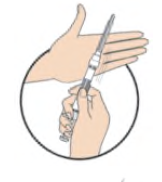 4. Hold the syringe UPRIGHT. With the opposite hand, pull the needle cap upward without twisting. 5. Keep the syringe UPRIGHT. Advance the plunger to expel the air from the syringe. 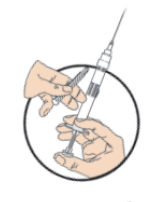 6. Inject the entire contents of the syringe at the time of the reconstitution. The suspension settles very quickly following reconstitution; therefore, leuprorelin acetate should be mixed and used immediately. Re-shake the suspension if settling occurs. 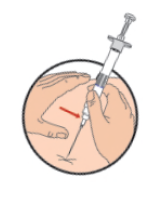 Note: Aspirated blood would be visible just below the luer lock connection if a blood vessel is accidentally penetrated. If present, blood can be seen through the transparent hub of the needle.
SUBCUTANEOUS, INTRAMUSCULAR
Medical Information
**INDICATIONS** _**Prostate Cancer**_ Leuprorelin acetate for depot suspension is indicated in the palliative treatment of advanced prostatic cancer. It offers an alternative treatment of prostatic cancer when orchiectomy or estrogen administration are either not indicated or unacceptable to the patient. In clinical trials, the safety and efficacy of leuprorelin acetate for depot suspension does not differ from that of the daily subcutaneous injection dosage. _**Endometriosis**_ Leuprorelin acetate for depot suspension is indicated in the treatment of endometriosis for a period of six months. It can be used as sole therapy or as an adjunct to surgery. Leuprorelin acetate for depot suspension with norethisterone 5mg daily as add-back is also indicated for treatment of endometriosis for a period of six months. **_Uterine Fibroids_** Leuprorelin acetate for depot suspension is also indicated in the treatment of leiomyoma uteri (uterine fibroids), as a pre-operative treatment only, for a period up to six months. _**Breast Cancer**_ Leuprorelin acetate for depot suspension is indicated for the treatment of breast cancer in pre- and peri-menopausal women in which hormone therapy is specified.
**CONTRAINDICATIONS** Leuprorelin acetate is contraindicated in patients with known hypersensitivity to leuprorelin acetate, similar nonapeptides, or any of the excipients. Isolated cases of anaphylaxis have been reported with the monthly formulation of leuprorelin acetate. Leuprorelin acetate is contraindicated in women who are or may become pregnant while receiving the drug. When administered on day 6 of pregnancy at test dosages of 0.00024, 0.0024, and 0.024 mg/kg (1/300 to 1/3\* of the human dose) to rabbits, leuprorelin acetate (Depot Formulation) produced a dose-related increase in major fetal abnormalities. Similar studies in rats failed to demonstrate an increase in fetal malformations. There was increased fetal mortality and decreased fetal weights with the two higher doses of the monthly formulation of leuprorelin acetate in rabbits and with the highest dose in rats. The effects on fetal mortality are logical consequences of the alterations in hormonal levels brought about by this drug. Therefore, the possibility exists that spontaneous abortion may occur if the drug is administered during pregnancy. **\\* NOTE:** the safety margin has been calculated based on the estimated average daily release of leuprorelin acetate from the depot formulation both for human and animals. An overall safety margin has been used that is expected to represent all of the leuprorelin acetate formulations worldwide adequately. Leuprorelin acetate should not be administered to patients with undiagnosed vaginal bleeding.
L02AE02
leuprorelin
Manufacturer Information
ABBVIE PTE. LTD.
Takeda Pharmaceutical Company Limited (Osaka Plant)
Takeda Pharmaceutical Company Limited (Hikari Plant)
Active Ingredients
Documents
Package Inserts
Lucrin Depot 11.25mg PI.pdf
Approved: October 15, 2018
