Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**4.2 Posology and method of administration** Treatment must be initiated and supervised by physicians experienced in the treatment of cancer. Posology _OPDIVO as monotherapy_ The recommended dose of OPDIVO is 3 mg/kg administered intravenously over 30–60 minutes every 2 weeks. _Oesophageal Squamous Cell Carcinoma (OSCC)_ The recommended dose of OPDIVO is 240 mg administered intravenously over 30–60 minutes every 2 weeks. _Adjuvant treatment of oesophageal or gastroesophageal junction cancer (OC or GEJC)_ The recommended dose of OPDIVO is 240 mg administered intravenously over 30–60 minutes every 2 weeks, or 480 mg administered intravenously over 30–60 minutes every 4 weeks. _Muscle invasive urothelial carcinoma (MIUC) adjuvant treatment_ The recommended dose of OPDIVO is 240 mg administered intravenously over 30–60 minutes every 2 weeks, or 480 mg administered intravenously over 30–60 minutes every 4 weeks. _OPDIVO in combination with ipilimumab_ _Melanoma_ The recommended dose is 1 mg/kg nivolumab administered as an intravenous infusion over 30–60 minutes every 3 weeks for the first 4 doses in combination with 3 mg/kg ipilimumab administered intravenously over 30–90 minutes. This is then followed by a second phase in which 3 mg/kg nivolumab is administered as an intravenous infusion over 30–60 minutes every 2 weeks. _Non-Small Cell Lung Cancer (NSCLC)_ The recommended dose is 360 mg nivolumab administered as an intravenous infusion every 3 weeks in combination with 1 mg/kg ipilimumab administered as an intravenous infusion every 6 weeks, and platinum chemotherapy administered every 3 weeks. After completion of 2 cycles of chemotherapy, treatment is continued with 360 mg nivolumab administered as an intravenous infusion every 3 weeks in combination with 1 mg/kg ipilimumab every 6 weeks. Treatment is recommended until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression. _Malignant pleural mesothelioma (MPM)_ The recommended dose of nivolumab is either 3 mg/kg every 2 weeks or 360 mg every 3 weeks administered as an intravenous infusion over 30–60 minutes in combination with 1 mg/kg ipilimumab administered as an intravenous infusion over 30 minutes every 6 weeks. Treatment is recommended until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression. _Renal Cell Carcinoma (RCC)_ The recommended dose is 3 mg/kg nivolumab administered as an intravenous infusion over 30–60 minutes every 3 weeks for the first 4 doses in combination with 1 mg/kg ipilimumab administered intravenously over 30 minutes. This is then followed by a second phase in which 3 mg/kg nivolumab is administered as an intravenous infusion over 30–60 minutes every 2 weeks. The first dose of nivolumab monotherapy should be administered 3 weeks following the last dose of the combination of nivolumab and ipilimumab. _Oesophageal squamous cell carcinoma_ The recommended dose is either 3 mg/kg nivolumab every 2 weeks or 360 mg nivolumab every 3 weeks administered intravenously over 30-60 minutes in combination with 1 mg/kg ipilimumab administered intravenously over 30 minutes every 6 weeks. Treatment is recommended until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression. _OPDIVO in combination with cabozantinib_ _Renal Cell Carcinoma (RCC)_ The recommended dose is nivolumab administered intravenously at either 240 mg every 2 weeks over 30 minutes or 480 mg every 4 weeks over 60 minutes in combination with 40 mg cabozantinib administered orally every day. _OPDIVO in combination with chemotherapy_ _Neoadjuvant treatment of non-small cell lung cancer_ The recommended dose is 360 mg nivolumab administered intravenously over 30–60 minutes in combination with platinum-based chemotherapy every 3 weeks for 3 cycles (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Gastric cancer, gastroesophageal junction cancer and oesophageal adenocarcinoma_ The recommended dose is 360 mg nivolumab administered intravenously over 30–60 minutes in combination with fluoropyrimidine- and platinum-based chemotherapy every 3 weeks or 240 mg nivolumab administered intravenously over 30–60 minutes in combination with fluoropyrimidine- and platinum-based chemotherapy every 2 weeks (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Treatment is recommended until disease progression or unacceptable toxicity. The maximum treatment duration for OPDIVO is 24 months. _Oesophageal squamous cell carcinoma_ The recommended dose of nivolumab is 240 mg every 2 weeks or 480 mg every 4 weeks administered intravenously over 30–60 minutes in combination with fluoropyrimidine- and platinum-based chemotherapy (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Treatment with nivolumab is recommended until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression. _Duration of treatment_ Treatment with OPDIVO, either as monotherapy or in combination with ipilimumab or other therapeutic agents, should be continued as long as clinical benefit is observed or until treatment is no longer tolerated by the patient (and up to maximum duration of therapy if specified for an indication). For adjuvant therapy, the maximum treatment duration with OPDIVO is 12 months. Atypical responses (i.e., an initial transient increase in tumour size or small new lesions within the first few months followed by tumour shrinkage) have been observed. It is recommended to continue treatment with nivolumab or nivolumab in combination with ipilimumab for clinically stable patients with initial evidence of disease progression until disease progression is confirmed. For OPDIVO in combination with cabozantinib, nivolumab should be continued until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression. Cabozantinib should be continued until disease progression or unacceptable toxicity. Refer to the product insert for cabozantinib. Dose escalation or reduction is not recommended for OPDIVO as monotherapy or in combination with other therapeutic agents. Dosing delay or discontinuation may be required based on individual safety and tolerability. Guidelines for permanent discontinuation or withholding of doses are described in Table 1. Detailed guidelines for the management of immune-related adverse reactions are described in section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. When nivolumab is administered in combination with other therapeutic agents, refer to the product insert of these other combination therapeutic agents regarding dosing. 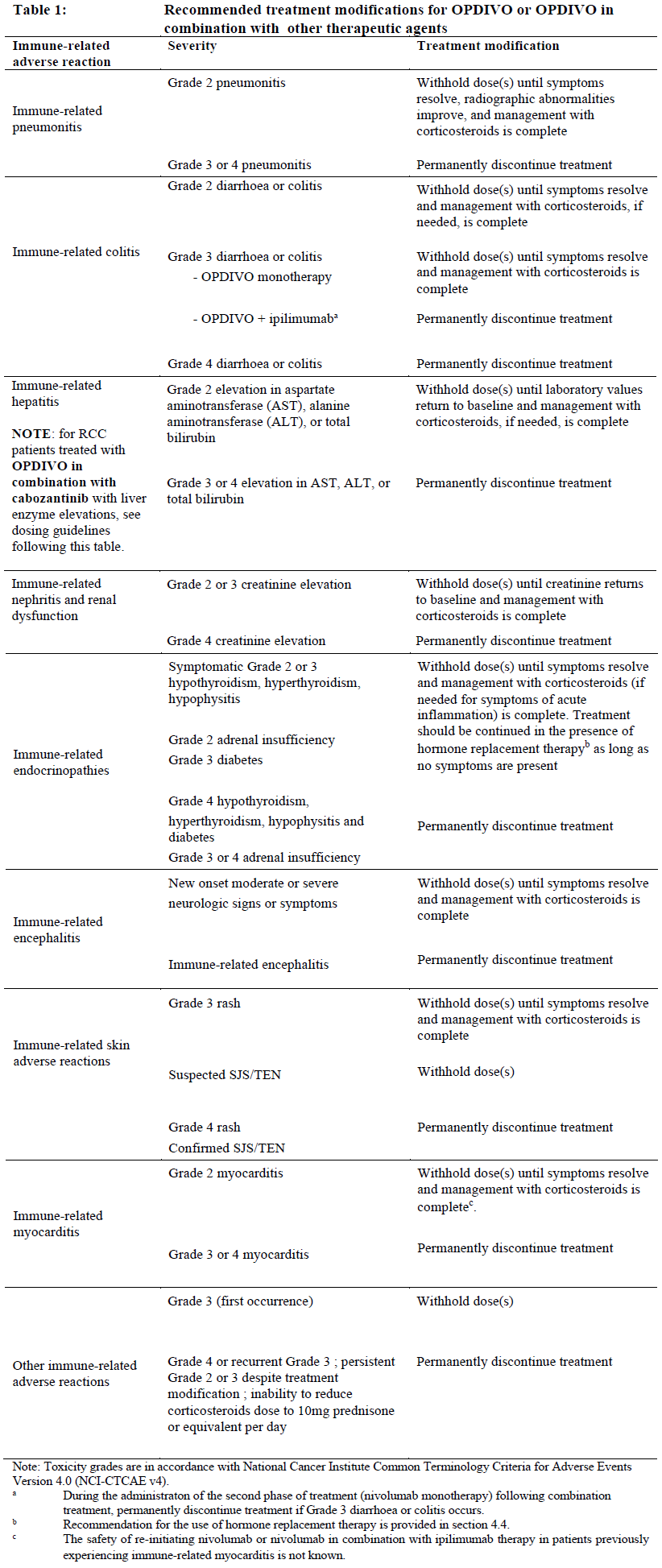 OPDIVO as monotherapy or in combination with other therapeutic agents should be permanently discontinued for: - Grade 4 or recurrent Grade 3 adverse reactions; - Persistent Grade 2 or 3 adverse reactions despite management. When OPDIVO is administered in combination with ipilimumab, if either agent is withheld, the other agent should also be withheld. If dosing is resumed after a delay, either the combination treatment or OPDIVO monotherapy could be resumed based on the evaluation of the individual patient. When OPDIVO is administered in combination with chemotherapy, refer to the product information of the other combination therapy agents regarding dosing. If any agents are withheld, the other agents may be continued. If dosing is resumed after a delay, either the combination treatment, OPDIVO monotherapy or chemotherapy alone could be resumed based on the evaluation of the individual patient. _OPDIVO in combination with cabozantinib in RCC_ When nivolumab is used in combination with cabozantinib, the above treatment modifications in Table 1 also apply to the nivolumab component. In addition, for liver enzyme elevations, in patients with RCC being treated with nivolumab in combination with cabozantinib: - If ALT or AST > 3 times ULN but ≤ 10 times ULN without concurrent total bilirubin ≥ 2 times ULN, both nivolumab and cabozantinib should be withheld until these adverse reactions recover to Grades 0–1. Corticosteroid therapy may be considered. Rechallenge with a single medicine or rechallenge with both medicines after recovery may be considered. If rechallenging with cabozantinib, refer to cabozantinib product insert. - If ALT or AST > 10 times ULN or > 3 times ULN with concurrent total bilirubin ≥ 2 times ULN, both nivolumab and cabozantinib should be permanently discontinued and corticosteroid therapy may be considered. For RCC patients treated with nivolumab in combination with cabozantinib, refer to the product insert regarding treatment modifications of cabozantinib. _Special populations_ _Paediatric population_ The safety and efficacy of OPDIVO in children below 18 years of age have not been established. No data are available. _Elderly_ No dose adjustment is required for elderly patients (≥ 65 years) (see sections 5.1 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ Based on the population pharmacokinetic (PK) results, no dose adjustment is required in patients with mild or moderate renal impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Data from patients with severe renal impairment are too limited to draw conclusions on this population. _Hepatic impairment_ Based on the population PK results, no dose adjustment is required in patients with mild hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Data from patients with moderate (total bilirubin > 1.5 x to 3 x the upper limits of normal \[ULN\] and any AST) or severe (total bilirubin >3 x ULN and any AST) hepatic impairment are too limited to draw conclusions on these populations. Method of administration OPDIVO is for intravenous use only. It is to be administered as an intravenous infusion over a period of 30– 60 minutes. The infusion must be administered through a sterile, non-pyrogenic, low protein binding in-line filter with a pore size of 0.2–1.2 micrometre. Do not coadminister other drugs through the same intravenous line. OPDIVO must not be administered as an intravenous push or bolus injection. The total dose of OPDIVO required can be infused directly as a 10 mg/mL solution or can be diluted to as low as 1 mg/mL with sodium chloride 9 mg/mL (0.9%) solution for injection or glucose 50 mg/mL (5%) solution for injection. When administered in combination with ipilimumab and/or chemotherapy, OPDIVO should be given first followed by ipilimumab (if applicable) and then chemotherapy on the same day. Use separate infusion bags and filters for each infusion. For instructions on the handling of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS DRIP
Medical Information
**4.1 Therapeutic indications** Melanoma OPDIVO as monotherapy or in combination with ipilimumab is indicated for the treatment of advanced (unresectable or metastatic) melanoma in adults. Relative to nivolumab monotherapy, an increase in progression-free survival (PFS) for the combination of nivolumab with ipilimumab is established only in patients with low tumour PD-L1 expression (see sections 4.4 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). OPDIVO as monotherapy is indicated for the adjuvant treatment of adults with melanoma with involvement of lymph nodes or metastatic disease who have undergone complete resection. Non-Small Cell Lung Cancer (NSCLC) OPDIVO, in combination with ipilimumab and 2 cycles of platinum-based chemotherapy, is indicated for the first-line treatment of metastatic or recurrent NSCLC in adult patients with no EGFR or ALK genomic tumor mutations. OPDIVO as monotherapy is indicated for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) after prior chemotherapy in adults. Neoadjuvant treatment of NSCLC OPDIVO, in combination with platinum-doublet chemotherapy, is indicated for the neoadjuvant treatment of adult patients with resectable (tumours ≥4 cm or node positive) NSCLC. Malignant pleural mesothelioma (MPM) OPDIVO, in combination with ipilimumab, is indicated for the first-line treatment of adult patients with unresectable malignant pleural mesothelioma. Renal Cell Carcinoma (RCC) OPDIVO, in combination with ipilimumab, is indicated for the treatment of patients with intermediate or poor-risk, previously untreated advanced renal cell carcinoma. OPDIVO as monotherapy is indicated for the treatment of advanced renal cell carcinoma after prior therapy in adults. OPDIVO in combination with cabozantinib is indicated for the first-line treatment of adult patients with advanced renal cell carcinoma (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Classical Hodgkin Lymphoma (cHL) OPDIVO as monotherapy is indicated for the treatment of adult patients with relapsed or refractory classical Hodgkin lymphoma after autologous stem cell transplant (ASCT) and treatment with brentuximab vedotin (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Squamous Cell Cancer of the Head and Neck (SCCHN) OPDIVO as monotherapy is indicated for the treatment of recurrent or metastatic squamous cell cancer of the head and neck in adults progressing on or after platinum-based therapy in adults (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Gastric/ Gastroesophageal Junction (GEJ) Cancer OPDIVO as monotherapy is indicated for the treatment of adult patients with unresectable locally advanced or recurrent gastric or gastroesophageal junction (GEJ) adenocarcinoma after two or more prior systemic therapies. Oesophageal Squamous Cell Carcinoma (OSCC) OPDIVO in combination with ipilimumab is indicated for the first-line treatment of adult patients with unresectable advanced, recurrent or metastatic oesophageal squamous cell carcinoma with tumour cell PD-L1 expression ≥1%. OPDIVO in combination with fluoropyrimidine- and platinum-based combination chemotherapy is indicated for the first-line treatment of adult patients with unresectable advanced, recurrent or metastatic oesophageal squamous cell carcinoma with tumour cell PD-L1 expression ≥1%. OPDIVO as monotherapy is indicated for the treatment of adult patients with unresectable advanced, recurrent or metastatic oesophageal squamous cell carcinoma after prior fluoropyrimidine- and platinum-based combination chemotherapy. Gastric Cancer, Gastroesophageal Junction (GEJ) Cancer or Oesophageal Adenocarcinoma OPDIVO, in combination with fluoropyrimidine- and platinum-based chemotherapy, is indicated for the treatment of patients with unresectable HER2 negative advanced or metastatic gastric cancer, gastroesophageal junction cancer, or oesophageal adenocarcinoma (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Adjuvant treatment of oesophageal or gastroesophageal junction cancer (OC or GEJC) OPDIVO as monotherapy is indicated for the adjuvant treatment of completely resected oesophageal or gastroesophageal junction cancer with residual pathologic disease in adult patients who have received neoadjuvant chemoradiotherapy (CRT). Urothelial carcinoma OPDIVO as monotherapy is indicated for the adjuvant treatment of adults with muscle invasive urothelial carcinoma (MIUC) with tumor cell PD-L1 expression ≥1% who are at high risk of recurrence after undergoing radical resection of MIUC.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01FF01
nivolumab
Manufacturer Information
BRISTOL-MYERS SQUIBB (SINGAPORE) PTE. LTD.
Bristol-Myers Squibb Holdings Pharma, Ltd. Liability Company
Vetter Pharma-Fertigung GmBH & Co. KG
Active Ingredients
Documents
Package Inserts
OPDIVO injection PI.pdf
Approved: February 22, 2023
