Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**2 DOSAGE AND ADMINISTRATION** **BAT is for intravenous use only.** **2.1 Dosage and Administration** - Each vial of BAT contains a minimum potency for serotypes A, B, C, D, E, F, and G antitoxin _\[see Dosage Forms and Strengths (3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. - For adult, pediatric, and infant patient groups, administer a dose of BAT according to Table 1. For details on pediatric dosing by body weight see Table 2. - Administer all BAT doses after dilution 1:10 in normal saline by slow intravenous infusion according to the varying infusion rates in Table 1. - Monitor vital signs throughout the infusion. If tolerated, the infusion rate can be increased incrementally up to the maximum infusion rate, and continued for the remainder of the administration. Decrease infusion rate if the patient develops discomfort or infusion-related adverse reactions. 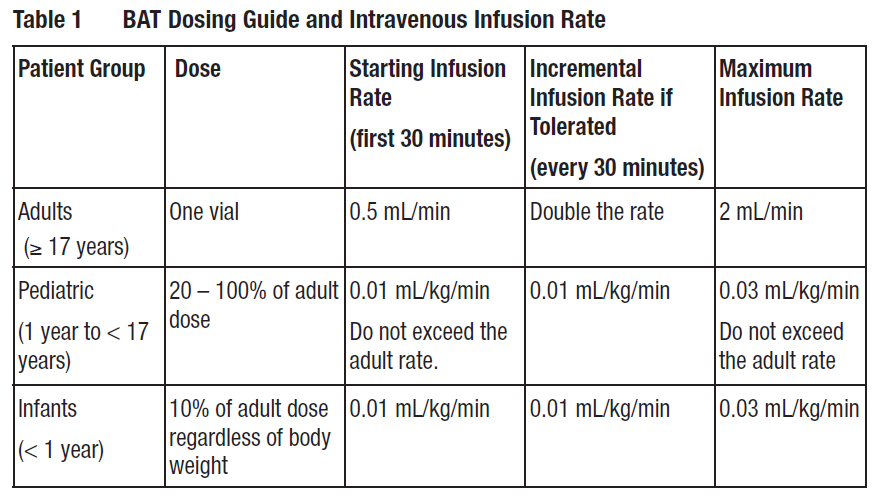 Calculate pediatric BAT dose by body weight according to Table 2. 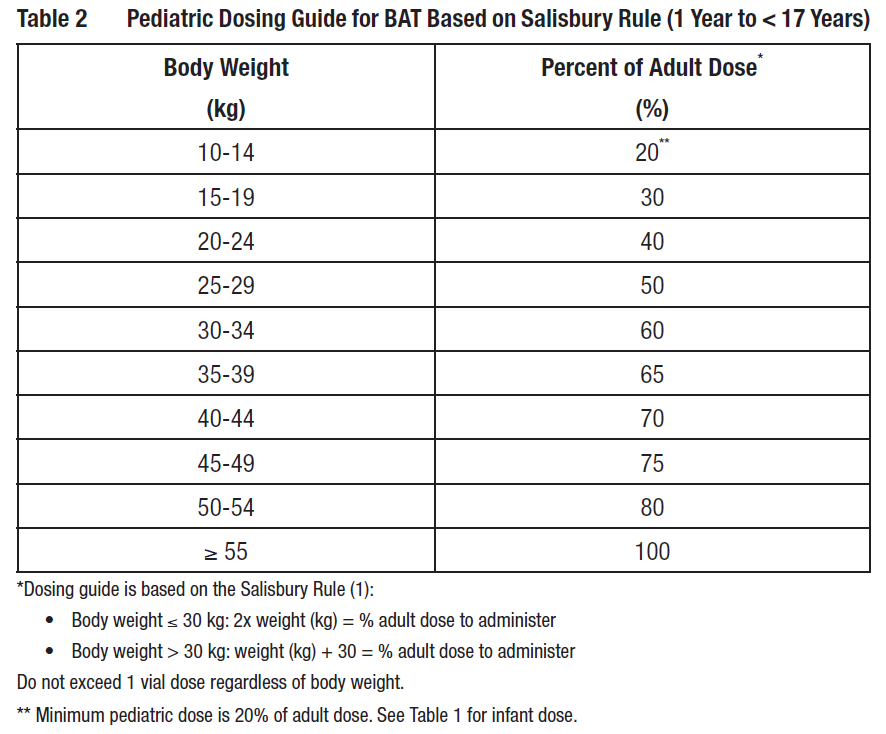 **2.2 Preparation** 1. Bring vial to room temperature. - If frozen, thaw vial by placing in a refrigerator at 36 to 46 °F (2 to 8 °C) until the contents are thawed for approximately 14 hours. - Product can be thawed rapidly by placing at room temperature for one hour followed by a water bath at 98.6 °F (37 °C) until thawed. - Do not thaw this product in a microwave oven. Do not refreeze the vial. 2. Inspect vial to ensure there is no damage to the seal or vial. If damaged, discard the vial. 3. Do not shake the vial during preparation to avoid foaming. 4. Dilute 1:10 in 0.9% Sodium Chloride Injection, USP (saline) by adding BAT solution from the vial to the appropriate amount of saline in an IV bag. Do not use any other diluents. As the fill volume per vial varies by lot number (ranging from 10 to 26 milliliters per vial), 90 to 235 milliliters of saline will be required. Withdraw the entire contents of the vial to obtain the total volume in the vial. If a partial vial is required (for pediatric dosing), the entire content of the vial should be withdrawn to ensure accurate calculation of the dosage \[Table 2\]. 5. Visually inspect the product for particulate matter and discoloration prior to administration. Do not use if the solution is turbid, cloudy, or contains particles other than a few translucent-to-white proteinaceous particulates. 6. Use an intravenous line with constant infusion pump. Use a 15 micron sterile, non-pyrogenic, low protein binding in-line filter. 7. BAT vials are for single use only and contain no preservative. Once punctured, use the vial contents to prepare the infusion bag and administer as soon as possible. 8. Discard any unused portion.
INTRAVENOUS
Medical Information
**1 INDICATIONS AND USAGE** BAT \[Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) – (Equine)\] is a mixture of immune globulin fragments indicated for the treatment of symptomatic botulism following documented or suspected exposure to botulinum neurotoxin serotypes A, B, C, D, E, F, or G in adults and pediatric patients. The effectiveness of BAT is based on efficacy studies conducted in animal models of botulism.
**4 CONTRAINDICATIONS** None.
J06AA04
botulinum antitoxin
Manufacturer Information
EMERGENT SALES AND MARKETING SINGAPORE PTE. LTD.
Emergent BioSolutions Canada Inc.
Active Ingredients
Botulism Antitoxin (Equine) Serotype G
> 600 U/vial
Botulism Antitoxin (Equine) Serotype B
> 3300 U/vial
Botulism Antitoxin (Equine) Serotype C
> 3000 U/vial
Botulism Antitoxin (Equine) Serotype A
> 4500 U/vial
Botulism Antitoxin (Equine) Serotype E
> 5100 U/vial
Botulism Antitoxin (Equine) Serotype D
> 600 U/vial
Botulism Antitoxin (Equine) Serotype F
> 3000 U/vial
Documents
Package Inserts
BAT STERILE SOLUTION FOR INJECTION PI.pdf
Approved: March 8, 2021
