Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2. Posology and method of administration** Treatment with MIRCERA has to be initiated under the supervision of a physician experienced in the management of patients with renal impairment. The solution can be administered subcutaneously or intravenously. MIRCERA can be injected subcutaneously in the abdomen, arm or thigh. All three injection sites are equally suitable. It is recommended that haemoglobin is monitored every two weeks until stabilized and periodically thereafter. As recommended in current guidelines, the rate of increase in Hb and the target Hb should be determined for each patient individually. In CKD patients, the aim of treatment is to reach a target Hb level of 10–12g/dL. Patients should be monitored closely to ensure that the lowest effective dose of MIRCERA is used to provide adequate control of the symptoms of anemia. Patients not currently treated with an erythropoiesis stimulating agent (ESA): The recommended starting dose is 0.6 microgram/kg body weight, administered once every two weeks as a single intravenous or subcutaneous injection. The dose may be increased by approximately 25% of the previous dose if the rate of rise in haemoglobin is less than 1.0 g/dl (0.621 mmol/l) over a month. Further increases of approximately 25% may be made at monthly intervals until the individual target haemoglobin level is obtained. If the rate of rise in haemoglobin is greater than 2 g/dl (1.24 mmol/l) in one month or the haemoglobin level is increasing and approaching 12 g/dl (7.45 mmol/l), the dose is to be reduced by approximately 25%. If the haemoglobin level continues to increase, therapy should be interrupted until the hemoglobin level begins to decrease, at which point therapy should be restarted at a dose approximately 25% below the previously administered dose. After dose interruption a haemoglobin decrease of approximately 0.35 g/dl per week is expected. Dose adjustments should not be made more frequently than once a month. If the target haemoglobin concentration level of 12 g/dl (7.45 mmol/l) is reached for the individual patient, MIRCERA may be administered once monthly using the dose equal to twice the previous once every two weeks dose. Patients currently treated with an ESA: Patients currently treated with an ESA can be converted to MIRCERA administered once a month as a single intravenous or subcutaneous injection. The starting dose of methoxy polyethylene glycol-epoetin beta is based on the calculated previous weekly dose of darbepoetin alfa or epoetin at the time of substitution as described in Table 1. The first injection should start at the next scheduled dose of the previously administered darbepoetin alfa or epoetin. 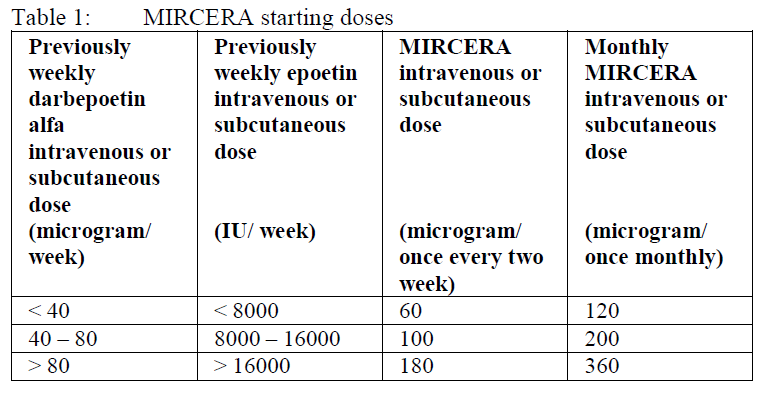 If a dose adjustment is required to maintain the target haemoglobin concentration of 10 g/dl, the monthly dose may be adjusted by approximately 25%. If the rate of rise in haemoglobin is greater than 2 g/dl (1.24 mmol/l) over a month or the haemoglobin level is increasing and approaching 12 g/dl (7.45 mmol/l), the dose is to be reduced by approximately 25%. If the haemoglobin level continues to increase, therapy should be interrupted until the hemoglobin level begins to decrease, at which point therapy should be restarted at a dose approximately 25% below the previously administered dose. After dose interruption a haemoglobin decrease of approximately 0.35 g/dl per week is expected. Dose adjustments should not be made more frequently than once a month. Since the treatment experience is limited in patients on peritoneal dialysis, regular Hb monitoring and strict adherence to dose adjustment guidance is recommended in these patients. Treatment interruption Treatment with MIRCERA is normally long-term. However, it can be interrupted at any time, if necessary. Missed dose If one dose of MIRCERA is missed, the missed dose is to be administered as soon as possible and administration of MIRCERA is to be restarted at the prescribed dosing frequency. Paediatric use MIRCERA is not recommended for use in children and adolescents below 18 years due to a lack of safety and efficacy data. Geriatric use In clinical studies 24% of patients treated with MIRCERA were age 65 to 74 years, while 20% were age 75 years and over. No dose adjustment is required in patients aged 65 years or older. Hepatic impaired patients No adjustments of the starting dose nor dose modification rules are required in patients with any degree of hepatic impairment (see section 5.4 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS, SUBCUTANEOUS
Medical Information
**4.1. Therapeutic indications** Treatment of anaemia associated with chronic kidney disease (CKD). The safety and efficacy of MIRCERA therapy in other indications has not been established, therefore such use is not recommended.
**4.3. Contraindications** Hypersensitivity to the active substance or any of the excipients. Patients with uncontrolled hypertension.
B03XA03
methoxy polyethylene glycol-epoetin beta
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
F. Hoffmann-La Roche AG
Active Ingredients
Documents
Package Inserts
Mircera Injection PI.pdf
Approved: April 10, 2019
