Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage and Administration(s):** - The total daily dose can range from 30 to 160 mg/kg/day depending on the indication. This is administered twice daily, but may also be given in three or four separate doses. - When treating severe symptoms, 12 g daily may need to be administered as an intravenous infusion. - In the treatment of chronic psycho-organic brain syndrome in the elderly 4.8 g daily is given initially, followed, after a few weeks, by maintenance therapy at 1.2 to 2.4 g per day. _Elderly_ Adjustment of the dose is recommended in elderly patients with compromised renal function _(see Sections: Precaution(s)/Warning(s)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _; Renal Impairment below)_. For long-term treatment in the elderly, regular evaluation of the creatinine clearance is required to allow dosage adaptation if needed. _Renal impairment_ Piracetam is contraindicated in severe renal impairment (renal creatinine clearance of less than 20 ml per minute) _(see Sections: Contraindications; Precaution(s)/Warning(s)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. The daily dose must be individualised according to renal function. Refer to the following table and adjust the dose as indicated. To use this dosing table, an estimate of the patient’s creatinine clearance (CLcr) in ml/min is needed. The CLcr in ml/min may be estimated from serum creatinine (mg/dl) determination using the following formula:  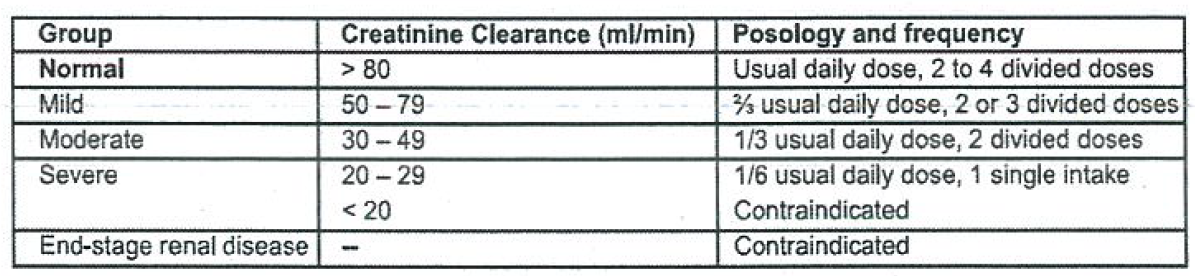 _Hepatic impairment_ No dose adjustment is needed in patients with solely hepatic impairment. In patients with hepatic impairment and renal impairment, adjustment of dose is recommended _(see dose adjustment in Renal Impairment above)_. **Mode of Administration:** Parenteral: For Intravenous Use.
INTRAVENOUS
Medical Information
**Indication(s):** Treatment of the elderly with some degree of cerebral functional impairment such as loss of memory, a lack of concentration or alertness and vertigo.
**Contraindication(s):** Piracetam is contraindicated in: - hypersensitivity to piracetam, other pyrrolidone derivatives or any of the excipients, - severe renal disease (renal creatinine clearance of less than 20 ml per minute), - cerebral haemorrhage, - Huntington’s chorea.
N06BX03
piracetam
Manufacturer Information
YUNG SHIN PHARMACEUTICAL (SINGAPORE) PTE LTD
Y.S.P. INDUSTRIES (M) SDN. BHD.
Active Ingredients
Documents
Package Inserts
Cetam Injection 200mgmL PI.pdf
Approved: November 30, 2020
