Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION (RADIOPHARMACEUTICAL)
**4.2 DOSE AND METHOD OF ADMINISTRATION** Posology If sodium pertechnetate (99mTc) is administered intravenously, activities may vary widely according to the clinical information required and the equipment employed. The injection of activities greater than local DRLs (Diagnostic Reference Levels) should be justified for certain indications. Recommended activities are as follows: Adults (70kg) and elderly population Thyroid scintigraphy: 20–80 MBq Renal impairment Careful consideration of the activity to be administered is required since an increased radiation exposure is possible in these patients. Paediatric population The use in children and adolescents has to be considered carefully, based upon clinical needs and assessing the risk/benefit ratio in this patient group. The activity to be administered to children and adolescents must be adapted <and may be calculated according to the recommendations of the European Association of Nuclear Medicine (EANM) paediatric dosage card>; the activity administered to children and to adolescents may be calculated by multiplying a baseline activity (for calculation purposes) by the weight-dependent correction factor given in the table below (see Table 6).  _Thyroid scintigraphy:_ Activity administered \[MBq\] = 5.6 MBq x correction factor (Table 6). A minimal activity of 10 MBq is necessary for obtaining images of sufficient quality. 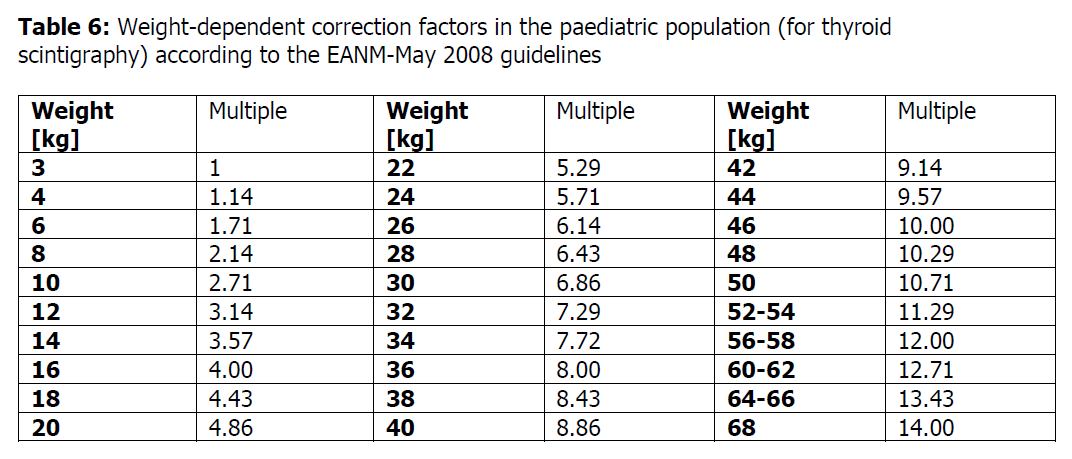 Method of administration For intravenous use. For multidose use. For instructions on extemporaneous preparation of the medicinal product before administration, see ‘Directions for Use’. For patient preparation, see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. In thyroid scintigraphy, the sodium pertechnetate (99mTc) solution is administered by intravenous injection. Image acquisition Thyroid scintigraphy: 20 minutes after intravenous injection. **GENTECH: DIRECTIONS FOR USE** **Gentech Molybdenum \[99Mo\]/Technetium \[99mTc\] Sterile Generator** The Gentech® generator is supplied with the following procedure packs –1) saline vials pack containing 0.9% sodium chloride injection BP (saline) vials and sterile wet wipes, and 2) evacuated vials pack containing evacuated elution vials, sterile wet wipes and needles. The generators are sterile and pyrogen-free when they leave ANSTO. To ensure the sterility of the eluate, aseptic techniques must be followed during elution of the Gentech®. Compliance to appropriate radiation safety regulations is required for handling generator eluate. **First Elution** 01. Remove the Gentech® generator and its accessories from the transport packaging. Install in the Gentech Garage or in the user shielding. 02. Lift Gentech® handle. Rotate the cover until the yellow saline spike cover and elution outlet filter are exposed. Push down handle to lock the lid in the operating position. 03. Remove flip off seal from saline vial (5 or 10 mL). The minimum elution volume is 5 mL. For elution volume between 5 and 10 mL, aseptically remove the unwanted saline from the vial with a hypodermic needle and discard. 04. Place Gentech® saline vial into the **new** Gentech® saline vial holder, provided in the foam insert of the transport package with every generator. Swab the exposed part of the saline vial’s silicone septum with a sterile swab provided. **Ensure to allow to dry.** 05. Remove the yellow protective cap from the Gentech® saline spike. 06. Align the lugs of the Gentech® saline vial holder with grooves in the saline port of the Gentech® generator and push down firmly. When vial is fully depressed, turn clockwise in direction of arrows to engage the vial on the saline spike and lock the saline vial holder in place. 07. Remove white plastic lid from the elution vial shield. Unscrew metal top. Remove the red flip-off seal from the 30 mL evacuated elution vial. Place the de-capped vial in the elution vial shield and screw on the metal cap to hold the vial in place. Swab the top of the evacuated elution vial shield and the exposed part of the septum of the evacuated elution vial, with a sterile swab provided. **Ensure to allow to dry.** 08. Grip the red protective cap (male luer closure), turn it anticlockwise through 90° and remove from the outlet filter. With the sterile needle cover in place, attach a sterile needle (screw clockwise). **Caution: do not over-tighten.** Remove the sterile needle cover. 09. Invert the prepared elution vial shield on to the sterile needle. Lower the elution vial shield until the evacuated vial is fully penetrated by the sterile needle. **Allow at least 3 minutes to complete the elution.** 10. Observe emptying of the saline vial and filling of the evacuated elution vial, indicated by the sight and sound of air bubbles in the elution vial. 11. Visibly check the saline vial is empty and through the elution vial shield window that the elution occurred. If elution did not occur, repeat steps 3 and 4 and 6 to 10 with a fresh saline and evacuated elution vials. 12. Remove the elution vial shield from the sterile needle. Cover the elution vial shield with white plastic lid. 13. Place the needle cover back on to the sterile needle and leave it in place until the next elution. (Replace with a fresh sterile needle before each elution). 14. **Do not remove saline vial assembly until the next elution.** 15. Record the appropriate information on the elution vial in accordance with your facility procedures, such as date, time and the contents being radioactive. 16. Assay the contents of the vial, for its 99mTc contents using a previously calibrated 99mTc dose calibrator (or other suitable measuring instrument). Calculate the total 99mTc content of the vial. Record the results. 17. Perform a gamma spectroscopy test to determine extent of 99Mo breakthrough. Alternate method described by \*Richards and O’Brien may be used. **Subsequent Elutions** 1. Remove the used saline vial (by twisting anti-clockwise), then repeat steps 3, 4, and 6, 7. 2. Remove used elution needle (by twisting anti-clockwise) and replace with a fresh sterile elution needle. 3. Repeat steps 9 through to 17. **Troubleshooting tips when the Generator is not eluting** 1. Check that the elution needle is not loose (see step 8). 2. Try another evacuated vial. 3. If you inadvertently remove the elution vial before it finishes eluting, the column will have become wet and will need to be dried. Attach a fresh evacuated vial but do not replace the saline vial unless it still contains some saline. In this case replace it with an empty saline vial. This process will allow air and not saline, to pass through and this will dry off the column. This process using an empty saline vial and a new evacuated elution vial can be repeated to ensure the column is dry. 4. Contact your local sales representative. **To Prevent Damaging the Spike** 1. Use a new Gentech® saline vial holder, provided with every new generator in the foam insert of the packaging of every new Gentech® generator. 2. Ensure the protective flip off seal is removed from the saline vial. 3. Ensure the lid of the Gentech® generator garage is fully open, to allow clear access to the Gentech® generator. 4. Ensure the yellow protective cap is removed from the saline spike. 5. Ensure the saline vial is placed on the spike vertically and not at an angle. 6. Following swabbing of the silicon septum of the saline vial, ensure to allow to dry. * * * _Reference: \*Richards, P. and O’Brien, M.J., Rapid determination of 99Mo in separated 99mTc. J. Nucl. Med., 10:517, 1969._
INTRAVENOUS
Medical Information
**4.1 THERAPEUTIC INDICATIONS** This product is for diagnostic use only. The eluate from the radionuclide generator (sodium pertechnetate \[99mTc\] solution for I.V. injection) is indicated for: - **labelling** of various kits for radiopharmaceutical preparation developed and approved for radiolabelling with such solution - **Thyroid scintigraphy:** direct imaging and measurement of thyroid uptake to give information on the size, position, nodularity and function of the gland in case of thyroid disease.
**4.3 CONTRAINDICATIONS** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
V09F X01
xv 09 f x 01
Manufacturer Information
TRANSMEDIC PTE LTD
ANSTO
Active Ingredients
Sodium pertechnetate (99mTc)
15.92/ 23.88/31.84/39.80/47.76/63.68/79.61/95.53 GBq
Documents
Package Inserts
Gentech PI.pdf
Approved: February 24, 2021
