Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**2\. DOSAGE AND ADMINISTRATION** **2.1 Recommended Dosage for Newly-Diagnosed Low-to-Intermediate-Risk Acute Promyelocytic Leukemia (APL)** A treatment course for patients with newly-diagnosed low-to-intermediate-risk APL consists of 1 induction cycle and 4 consolidation cycles. - For the induction cycle, the recommended dosage of Arsenic Trioxide is 0.15 mg/kg intravenously daily in combination with tretinoin until bone marrow remission but not to exceed 60 days (see Table 1). - For the consolidation cycles, the recommended dosage of Arsenic Trioxide is 0.15 mg/kg intravenously daily 5 days per week during weeks 1–4 of each 8-week cycle for a total of 4 cycles in combination with tretinoin (see Table 1). Omit tretinoin during weeks 5–6 of the fourth cycle of consolidation. 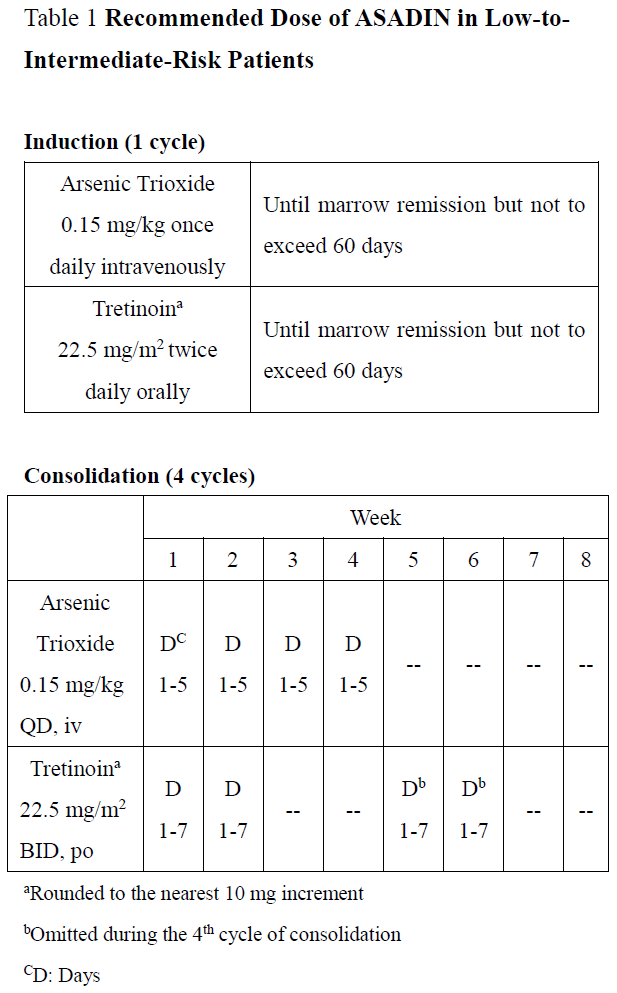 Differentiation syndrome prophylaxis consisting of prednisone 0.5 mg/kg daily from day 1 until the end of induction therapy with Arsenic Trioxide and tretinoin is recommended. **2.2 Recommended Dosage for Relapsed or Refractory APL** A treatment course for patients with relapsed or refractory APL consists of 1 induction cycle and 1 consolidation cycle \[see Clinical Studies (14.2) – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. - For the induction cycle, the recommended dosage of Arsenic Trioxide is 0.15 mg/kg/day intravenously daily until bone marrow remission or up to a maximum of 60 days. - For the consolidation cycle, the recommended dosage of Arsenic Trioxide is 0.15 mg/kg/day intravenously daily for 25 doses over a period of up to 5 weeks. Begin consolidation 3 to 6 weeks after completion of induction cycle. **2.3 Monitoring and Dosage Modifications for Adverse Reactions** During induction, monitor coagulation studies, blood counts, and chemistries at least 2–3 times per week through recovery. During consolidation, monitor coagulation studies, blood counts, and chemistries at least weekly. Table 2 shows the dose modifications for adverse reactions due to Arsenic Trioxide when used alone or in combination with tretinoin.  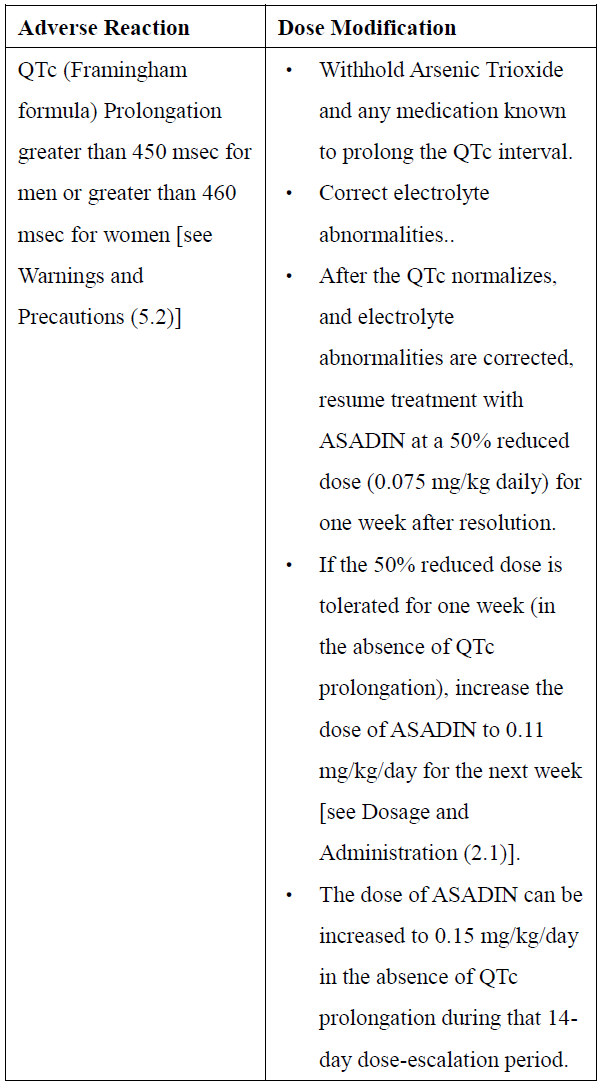 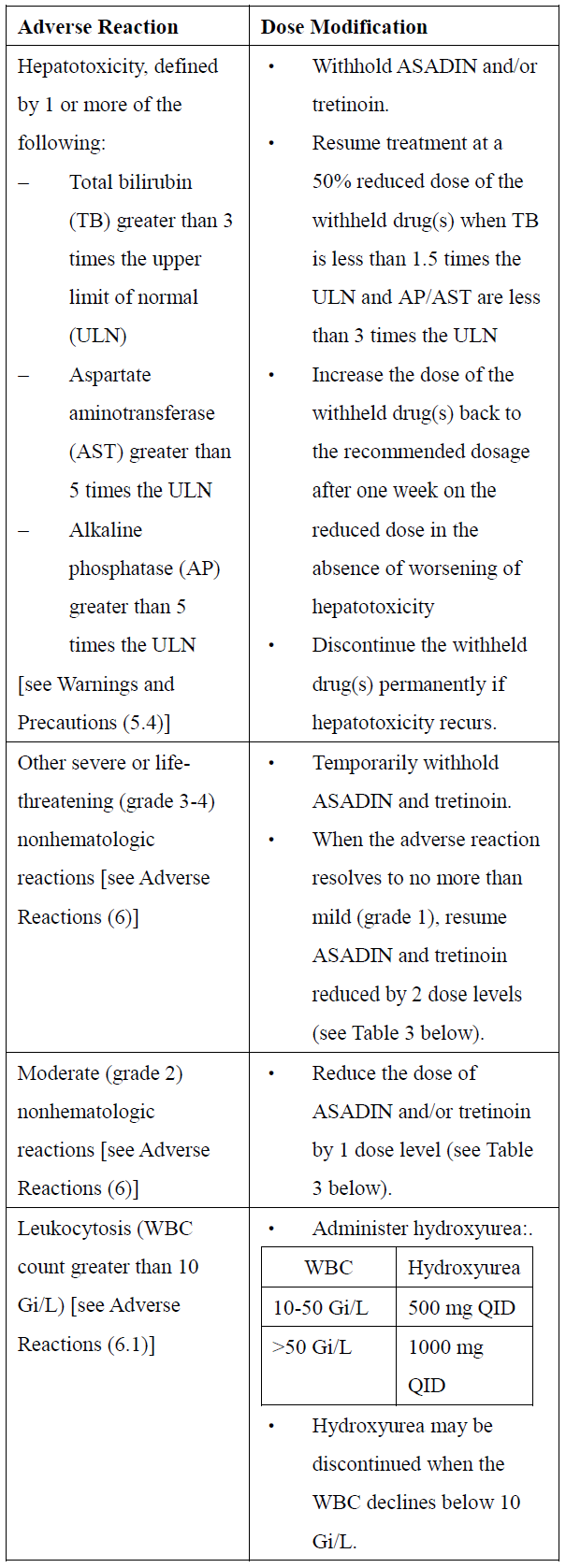 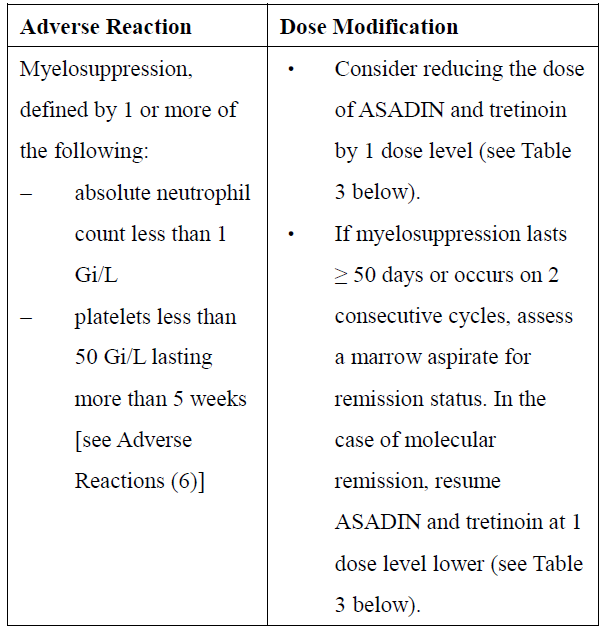 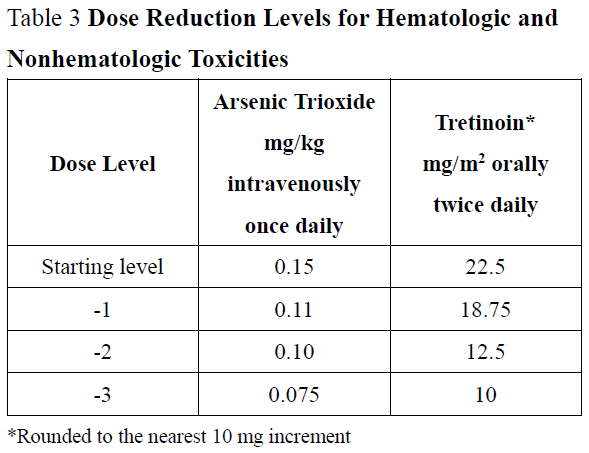 **2.4 Preparation and Administration** Reconstitution Dilute ASADIN with 100 or 250 mL 5% Dextrose Injection, or 0.9% Sodium Chloride Injection, using proper aseptic technique, immediately after withdrawal from the vial. After dilution, ASADIN is chemically and physically stable when stored for 24 hours at room temperature or 48 hours when refrigerated at 2–8°C. Administration Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Administer Arsenic Trioxide as an intravenous infusion over 2 hours. The infusion duration may be extended up to 4 hours if acute vasomotor reactions are observed. A central venous catheter is not required. The ASADIN vial is single-dose and does not contain any preservatives. Discard unused portions of each vial properly. Do not mix ASADIN with other medications. Safe Handling Procedures ASADIN is a cytotoxic drug. Follow applicable special handling and disposal procedures.
INTRAVENOUS
Medical Information
**1\. INDICATIONS AND USAGE** **1.1. Newly Diagnosed Low-to-Intermediate-Risk Acute promyelocytic leukaemia** Arsenic Trioxide is indicated in combination with tretinoin for treatment of adults with newly-diagnosed low-to-intermediate risk acute promyelocytic leukemia (APL) whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression. **1.2. Relapsed or Refractory Acute promyelocytic leukaemia** For the induction of remission and consolidation in patients with acute promyelocytic leukaemia (APL) who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterised by the presence of the t(15:17) translocation or PML/RAR alpha gene expression.
**4\. CONTRAINDICATIONS** ASADIN is contraindicated in patients with hypersensitivity to arsenic.
L01XX27
arsenic trioxide
Manufacturer Information
PHARM-D SINGAPORE PRIVATE LIMITED
TTY Biopharm Company Limited Chungli Factory
Active Ingredients
Documents
Package Inserts
Asadin Approved PI.pdf
Approved: November 2, 2022
