Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**2 DOSAGE AND ADMINISTRATION** In patients with a history or presence of clinically relevant central nervous system (CNS) pathology _\[see Warnings and Precautions (5.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_, hospitalization is recommended at a minimum for the first 14 days of the first cycle. In the second cycle, hospitalization is recommended at a minimum for 2 days, and clinical judgment should be based on tolerance to BLINCYTO in the first cycle. Caution should be exercised as cases of late occurrence of first neurological events in the second cycle have been observed. For all subsequent cycle starts and reinitiation (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended. **2.1 Treatment of MRD-positive B-cell Precursor ALL** - A treatment course consists of 1 cycle of BLINCYTO for induction followed by up to 3 additional cycles for consolidation. - A single cycle of treatment of BLINCYTO induction or consolidation consists of 28 days of continuous intravenous infusion followed by a 14-day treatment-free interval (total 42 days). - See Table 1 for the recommended dose by patient weight and schedule. Patients weighing 45 kg or more receive a fixed-dose. For patients weighing less than 45 kg, the dose is calculated using the patient’s body surface area (BSA). 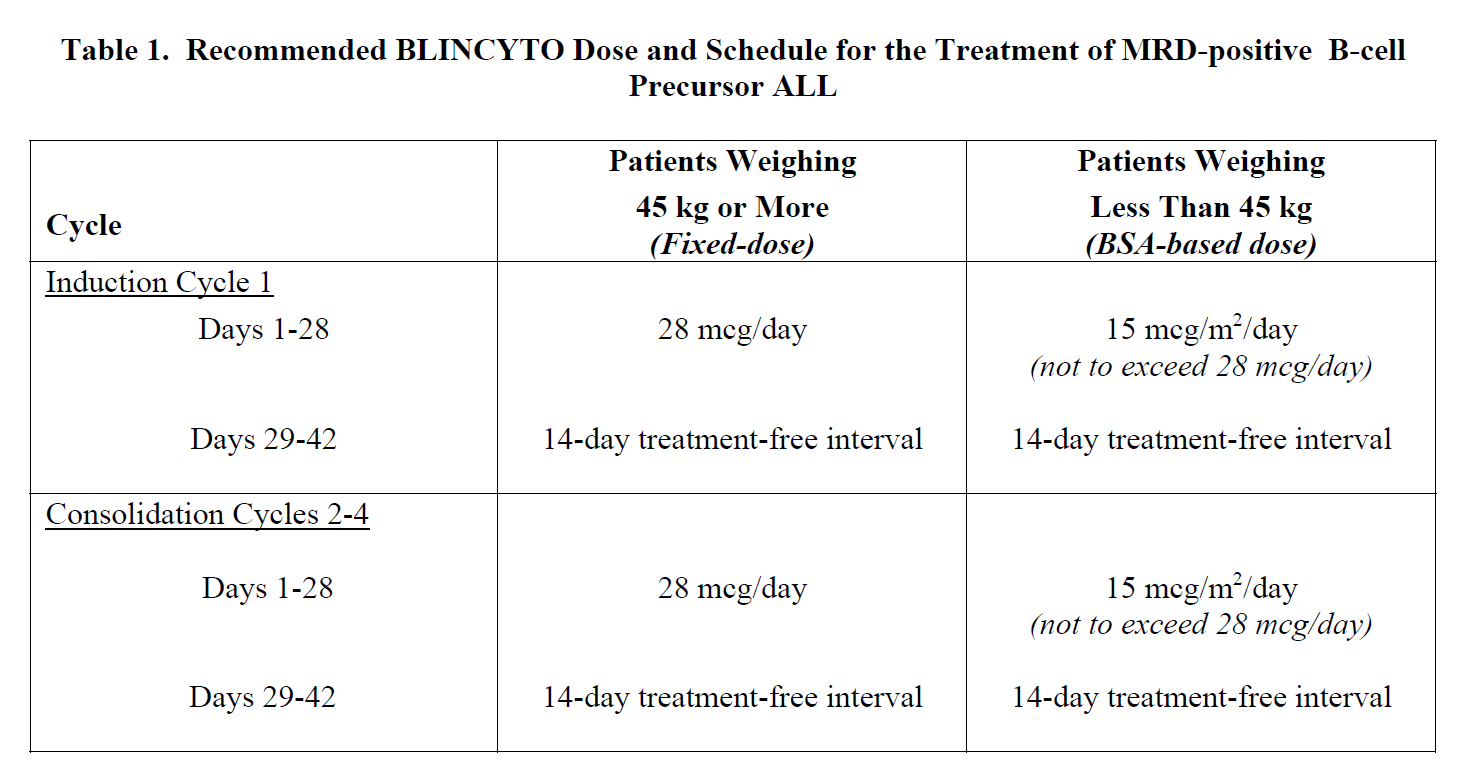 - Hospitalization is recommended for the first 3 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and re-initiations (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended. - Premedicate with prednisone or equivalent for MRD-positive B-cell Precursor ALL - For adult patients, premedicate with prednisone 100 mg intravenously or equivalent (e.g., dexamethasone 16 mg) 1 hour prior to the first dose of BLINCYTO in each cycle. - For pediatric patients, premedicate with 5 mg/m2 of dexamethasone, to a maximum dose of 20 mg, prior to the first dose of BLINCYTO in the first cycle and when restarting an infusion after an interruption of 4 or more hours in the first cycle. - For administration of BLINCYTO: - See Section 2.5 for infusion over 24 hours ,48 hours, 72 hours, or 96 hours. **2.2 Treatment of Relapsed or Refractory B-cell Precursor ALL** - A treatment course consists of up to 2 cycles of BLINCYTO for induction followed by 3 additional cycles for consolidation and up to 4 additional cycles of continued therapy. - A single cycle of treatment of BLINCYTO induction or consolidation consists of 28 days of continuous intravenous infusion followed by a 14-day treatment-free interval (total 42 days). - A single cycle of treatment of BLINCYTO continued therapy consists of 28 days of continuous intravenous infusion followed by a 56-day treatment-free interval (total 84 days). - See Table 2 for the recommended dose by patient weight and schedule. Patients weighing 45 kg or more receive a fixed-dose and for patients weighing less than 45 kg, the dose is calculated using the patient’s body surface area (BSA). 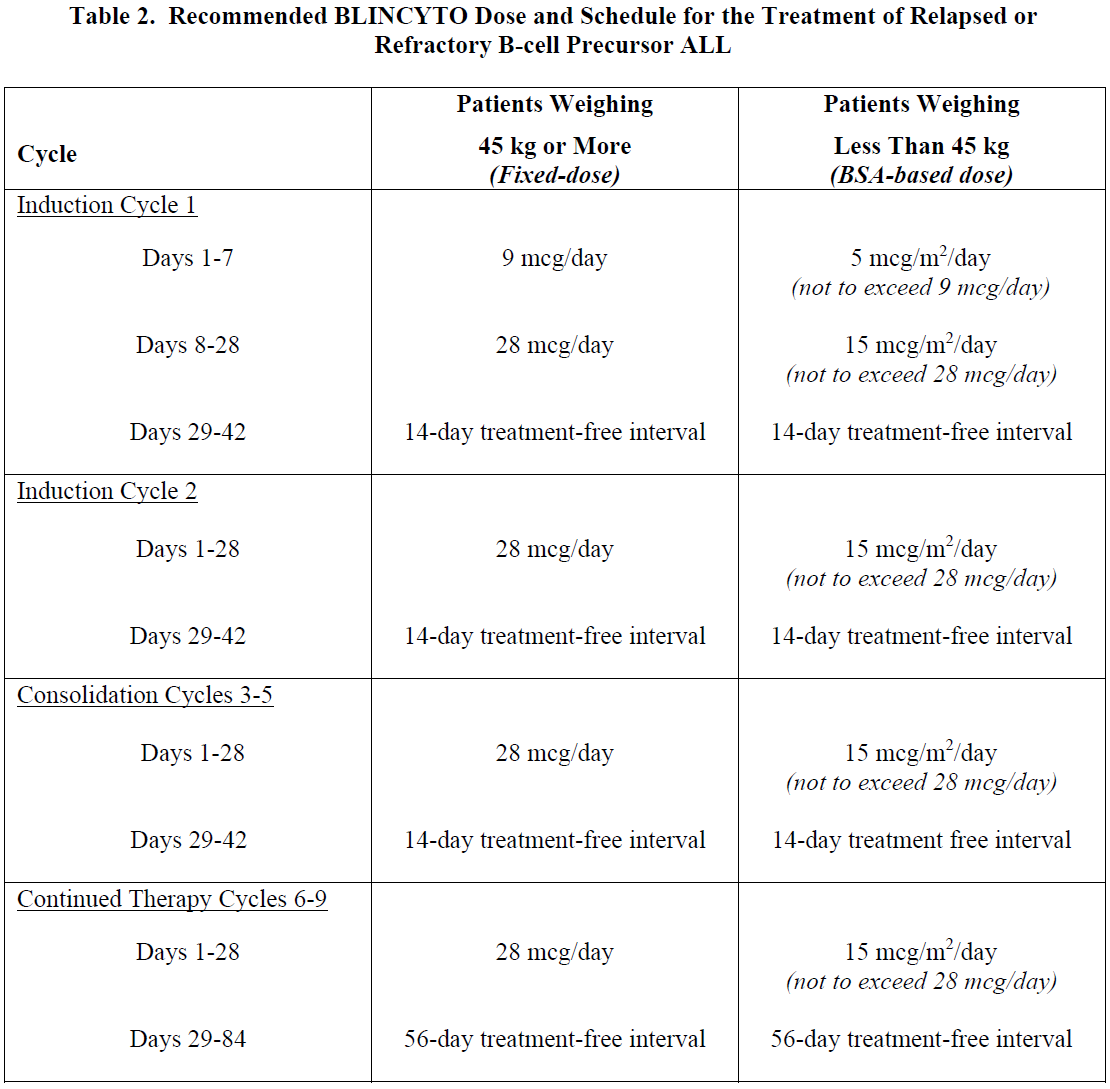 - Hospitalization is recommended for the first 9 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and re-initiation (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended. - Premedicate with dexamethasone. - For adult patients, premedicate with 20 mg of dexamethasone intravenously 1 hour prior to the first dose of BLINCYTO of each cycle, prior to a step dose (such as Cycle 1 Day 8), and when restarting an infusion after an interruption of 4 or more hours. - For pediatric patients, premedicate with 5 mg/m2 dexamethasone, to a maximum dose of 20 mg, prior to the first dose of BLINCYTO in the first cycle, prior to a step dose (such as Cycle 1 Day 8), and when restarting an infusion after an interruption of 4 or more hours in the first cycle. - For administration of BLINCYTO: - See Section 2.5 for infusion over 24 hours, 48 hours, 72 hours or 96 hours. - Pre-phase treatment for patients with high tumour burden - For pediatric and adult patients with > 50% leukemic blasts in bone marrow or > 15 X 109/L peripheral blood leukemic blast counts, treatment with dexamethasone (not to exceed 24 mg/day) for up to 4 days prior to the first dose of Blincyto is recommended. **2.3 Dosage Modifications for Adverse Reaction** If the interruption after an adverse reaction is no longer than 7 days, continue the same cycle to a total of 28 days of infusion inclusive of days before and after the interruption in that cycle. If an interruption due to an adverse reaction is longer than 7 days, start a new cycle. 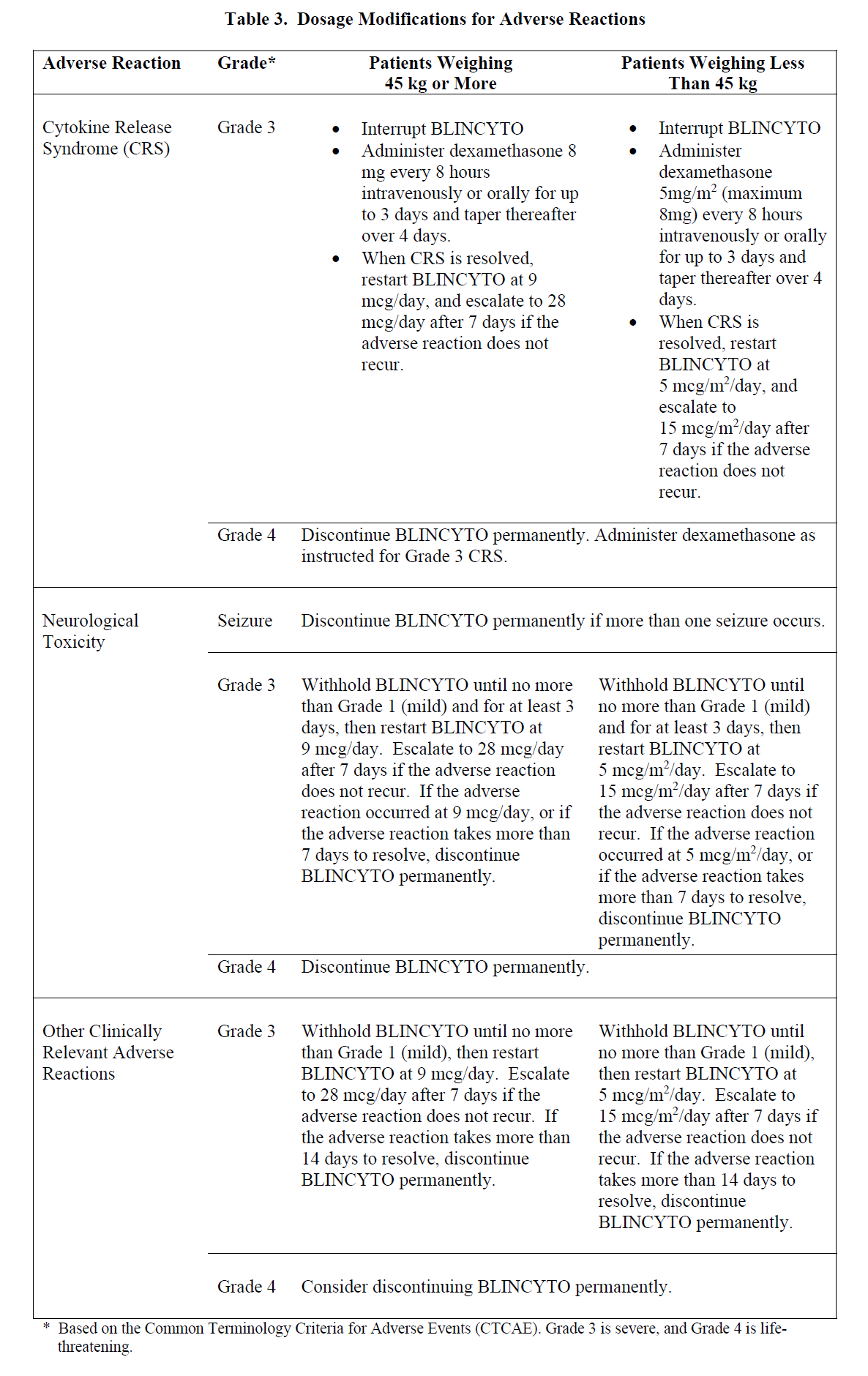 **2.4 Preparation** **It is very important that the instructions for preparation (including admixing) and administration provided in this section are strictly followed to minimize medication errors (including underdose and overdose)** _\[see Warnings and Precautions (5.13)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. BLINCYTO can be infused over 24 hours, 48 hours, 72 hours or 96 hours. The choice between these options for the infusion duration should be made by the treating healthcare provider considering the frequency of the infusion bag changes and the weight of the patient. For preparation, reconstitution, and administration of BLINCYTO: - See Section 2.5 for infusion over 24 hours, 48 hours, 72 hours or 96 hours. **2.4.1 Aseptic Preparation** Strictly observe aseptic technique when preparing the solution for infusion since BLINCYTO vials do not contain antimicrobial preservatives. To prevent accidental contamination, prepare BLINCYTO according to aseptic standards, including but not limited to: - Prepare BLINCYTO in a USP <797> compliant facility. - Prepare BLINCYTO in an ISO Class 5 laminar flow hood or better. - Ensure that the admixing area has appropriate environmental specifications, confirmed by periodic monitoring. - Ensure that personnel are appropriately trained in aseptic manipulations and admixing of oncology drugs. - Ensure that personnel wear appropriate protective clothing and gloves. - Ensure that gloves and surfaces are disinfected. **2.4.2 Package Content** 1 package BLINCYTO includes 1 vial of BLINCYTO and 1 vial of IV Solution Stabilizer. - **Do not use IV Solution Stabilizer for reconstitution of BLINCYTO.** IV Solution Stabilizer is provided with the BLINCYTO package and is used to coat the intravenous bag prior to addition of reconstituted BLINCYTO to prevent adhesion of BLINCYTO to intravenous bags and intravenous tubing. - More than 1 package of BLINCYTO may be needed to prepare the recommended dose. **2.4.3 Incompatibility Information** BLINCYTO is incompatible with di-ethylhexylphthalate (DEHP) due to the possibility of particle formation, leading to a cloudy solution. - Use polyolefin, DEHP-free PVC, or ethyl vinyl acetate (EVA) infusion bags/pump cassettes. - Use polyolefin, DEHP-free PVC, or EVA intravenous tubing sets. **2.5 Preparation and Administration of BLINCYTO as a 24-Hour, 48-Hour, 72-Hour or 96-Hour Infusion** **Reconstitute BLINCYTO with preservative-free Sterile Water for Injection, USP.** Do not reconstitute BLINCYTO vials with the IV Solution Stabilizer. **To prime the intravenous tubing, use only the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion.** Do not prime with 0.9% Sodium Chloride Injection, USP. 2.5.1 Reconstitution of BLINCYTO for 24-Hour, 48-Hour, 72-Hour or 96-Hour Infusion 1. Determine the number of BLINCYTO vials needed for a dose and infusion duration. 2. Reconstitute each BLINCYTO vial with **3 mL of preservative-free Sterile Water for Injection, USP** by directing the water along the walls of the BLINCYTO vial and not directly on the lyophilized powder. The resulting concentration per BLINCYTO vial is 12.5 mcg/mL. - Do not reconstitute BLINCYTO vials with IV Solution Stabilizer. 3. **Gently swirl contents to avoid excess foaming.** - Do not shake. 4. **Visually inspect the reconstituted solution for particulate matter and discoloration during reconstitution and prior to infusion.** The resulting solution should be clear to slightly opalescent, colorless to slightly yellow. - Do not use if solution is cloudy or has precipitated. 2.5.2 Preparation of BLINCYTO Infusion Bag for 24-Hour, 48-Hour, 72-Hour or 96-Hour Infusion Verify the prescribed dose and infusion duration for each BLINCYTO infusion bag. To minimize errors, **use the specific volumes described in Tables 4 and 5 to prepare the BLINCYTO infusion bag.** - Table 4 for patients weighing 45 kg or more - Table 5 for patients weighing less than 45 kg 1. Aseptically **add 270 mL 0.9% Sodium Chloride Injection, USP** to the empty intravenous bag. 2. Aseptically **transfer 5.5 mL IV Solution Stabilizer** to the intravenous bag containing 0.9% Sodium Chloride Injection, USP. Gently mix the contents of the bag to avoid foaming. Discard the vial containing the unused IV Solution Stabilizer. 3. Aseptically **transfer the required volume of reconstituted BLINCYTO solution** into the intravenous bag containing 0.9% Sodium Chloride Injection, USP and IV Solution Stabilizer. Gently mix the contents of the bag to avoid foaming. - Refer to Table 4 for patients weighing 45 kg or more for the specific volume of reconstituted BLINCYTO. - Refer to Table 5 for patients weighing less than 45 kg (dose based on BSA) for the specific volume of reconstituted BLINCYTO. - Discard the vial containing unused BLINCYTO. 4. Under aseptic conditions, attach the intravenous tubing to the intravenous bag with the sterile 0.2 micron in-line filter. Ensure that the intravenous tubing is compatible with the infusion pump. 5. Remove air from the intravenous bag. This is particularly important for use with an ambulatory infusion pump. 6. **Prime the intravenous tubing only with the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion.** 7. Store refrigerated at 2°C to 8°C if not used immediately _\[see Dosage and Administration (2.7)\]_. 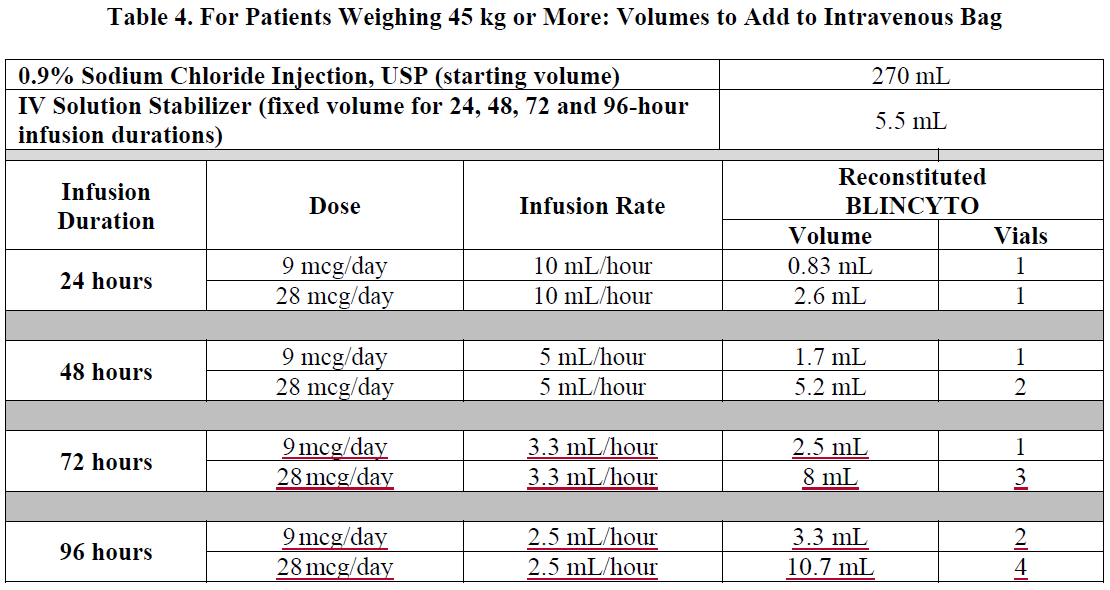 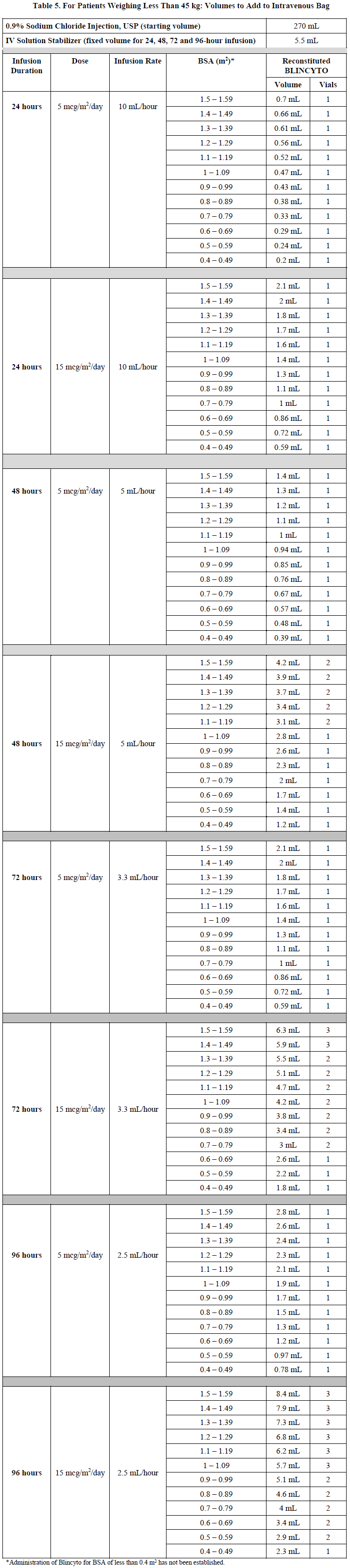 2.5.3 Administration of BLINCYTO for 24 Hour, 48-Hour, 72-Hour or 96-Hour Infusion - Administer BLINCYTO as a continuous intravenous infusion at a constant flow rate using an infusion pump. The pump should be programmable, lockable, non-elastomeric, and have an alarm. - The starting volume (270 mL) is more than the volume administered to the patient (240 mL) to account for the priming of the intravenous tubing and to ensure that the patient will receive the full dose of BLINCYTO. - Infuse prepared BLINCYTO final infusion solution according to the instructions on the pharmacy label on the prepared bag at one of the following constant infusion rates: - Infusion rate of 10 mL/hour for a duration of 24 hours, OR - Infusion rate of 5 mL/hour for a duration of 48 hours - Infusion rate of 3.3 mL/hour for a duration of 72 hours - Infusion rate of 2.5 mL/hour for a duration of 96 hours - Administer prepared BLINCYTO final infusion solution using intravenous tubing that contains a sterile, non-pyrogenic, low protein-binding, 0.2 micron in-line filter. - **Important Note: Do not flush the BLINCYTO infusion line or intravenous catheter, especially when changing infusion bags. Flushing when changing bags or at completion of infusion can result in excess dosage and complications thereof. When administering via a multi-lumen venous catheter, infuse BLINCYTO through a dedicated lumen.** - At the end of the infusion, discard any unused BLINCYTO solution in the intravenous bag and intravenous tubing in accordance with local requirements. **2.6 Storage Requirements of Reconstituted BLINCYTO** The information in Table 6 indicates the storage time for the reconstituted BLINCYTO vial and prepared infusion bag. 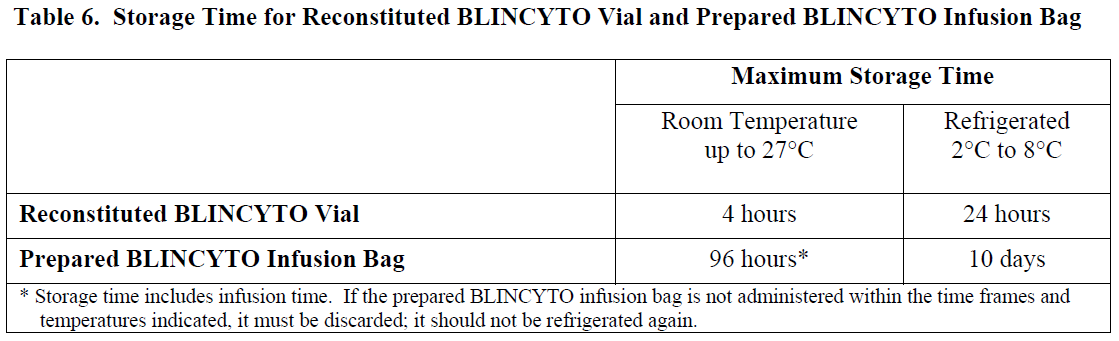
INTRAVENOUS
Medical Information
**1 INDICATIONS AND USAGE** **1.1 MRD-positive B-cell Precursor ALL** BLINCYTO is indicated for the treatment of B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1% in adult and pediatric patients. **1.2 Relapsed or Refractory B-cell Precursor ALL** BLINCYTO is indicated for the treatment of relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) in adult and pediatric patients.
**4 CONTRAINDICATIONS** BLINCYTO is contraindicated in patients with known hypersensitivity to blinatumomab or to any component of the product formulation.
L01XC19
xl 01 xc 19
Manufacturer Information
AMGEN BIOTECHNOLOGY SINGAPORE PTE. LTD.
Boehringer Ingelheim Pharma GmbH & Co. KG
Amgen Technology (Ireland) Unlimited Company
Active Ingredients
Documents
Package Inserts
BLINCYTO POWDER FOR INFUSION 35 MCG per VIAL PI.pdf
Approved: October 12, 2022
