Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**DOSAGE AND ADMINISTRATION** Gabapentin is given orally with or without food. When in the judgment of the clinician, there is a need for dose reduction, discontinuation, or substitution with an alternative medication, this should be done gradually over a minimum of 1 week. **Epilepsy** _**Adults and pediatric patients older than 12 years of age:**_ In clinical trials, the effective dosing range was 900mg/day to 1800mg/day. Therapy may be initiated by administering 300mg three times a day on Day 1, or by titrating the dose (Table 1). Thereafter, the dose can be increased in three equally divided doses up to a maximum dose of 1800 mg/day. Doses up to 2400mg/day have been well tolerated in long-term open-label clinical studies. Doses up to 3600mg/day have been administered to a small number of patients for a relatively short duration, and have been well tolerated. The maximum time between doses in the three times a day schedule should not exceed 12 hours to prevent breakthrough convulsions. 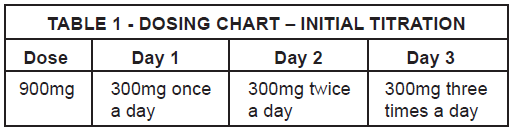 _**Pediatric patients aged 3 to 12 years:**_ The starting dose should range from 10 to 15 mg/kg/day given in equally divided doses (three times a day), and the effective dose reached by upward titration over a period of approximately 3 days. The effective dose of gabapentin in pediatric patients aged 5 years and older is 25 to 35 mg/kg/day given in equally divided doses (three times a day). The effective dose in pediatric patients aged 3 to less than 5 years is 40 mg/kg/day given in equally divided doses (three times a day). Doses up to 50 mg/kg/day have been well tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. **Neuropathic pain in adults** The starting dose is 900 mg/day given in three equally divided doses, and increased if necessary, based on response, up to a maximum dose of 3600 mg/day. Therapy should be initiated by titrating the dose (Table 1). **Dose adjustment in impaired renal function in patients with neuropathic pain or epilepsy** Dose adjustment is recommended in patients with compromised renal function (Table 2) and/or in those undergoing hemodialysis. 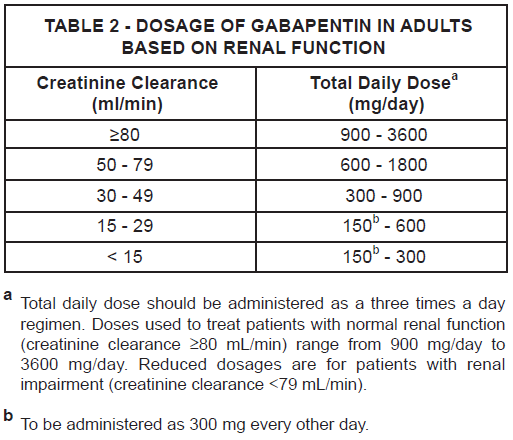 **Dose adjustment in patients undergoing hemodialysis** For patients undergoing hemodialysis who have never received gabapentin, a loading dose of 300 mg to 400 mg is recommended, and then 200 mg to 300 mg of gabapentin following each 4 hours of hemodialysis.
ORAL
Medical Information
**INDICATIONS** **Epilepsy:** Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 3 years and above. Safety and effectiveness for adjunctive therapy in pediatric patients below the age of 3 years have not been established. **Neuropathic Pain:** Gabapentin is indicated for the treatment of neuropathic pain which includes diabetic neuropathy, post-herpetic neuralgia and trigeminal neuralgia in adults age 18 years and above. Safety and effectiveness in patients below the age of 18 years have not been established.
**CONTRAINDICATIONS** Gabapentin is contraindicated in patients who are hypersensitive to gabapentin or the product’s components.
N03AX12
xn 03 ax 12
Manufacturer Information
GOLDPLUS UNIVERSAL PTE LTD
Hovid Bhd.
Active Ingredients
Documents
Package Inserts
Neuran 300mg PI.pdf
Approved: June 20, 2022
