Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2 DOSAGE AND ADMINISTRATION** **2.1 Recommended Dosage** The recommended dose of TUKYSA is 300 mg (two 150 mg tablets) taken orally twice daily until disease progression or unacceptable toxicity. TUKYSA tablets should be swallowed whole. Tablets should not be chewed, crushed, or split prior to swallowing. TUKYSA should be taken approximately 12 hours apart, at the same time every day, with or without a meal. In the case of a missed dose, the next dose should be taken at the regularly scheduled time. Refer to the Prescribing Information for trastuzumab administered in combination with TUKYSA for recommended dosing. When given with TUKYSA, the recommended dose of capecitabine is 1000 mg/m2 orally twice daily on Days 1 to 14 every 21 days, taken within 30 minutes after a meal. TUKYSA may be taken at the same time with capecitabine. **2.2 Dose Modifications** Dose Modifications for Adverse Reactions The recommended TUKYSA dose modifications for patients with adverse reactions are provided in Tables 1 to 4. Refer to the Full Prescribing Information for co-administered trastuzumab and capecitabine for dose modifications for toxicities suspected to be caused by those therapies. 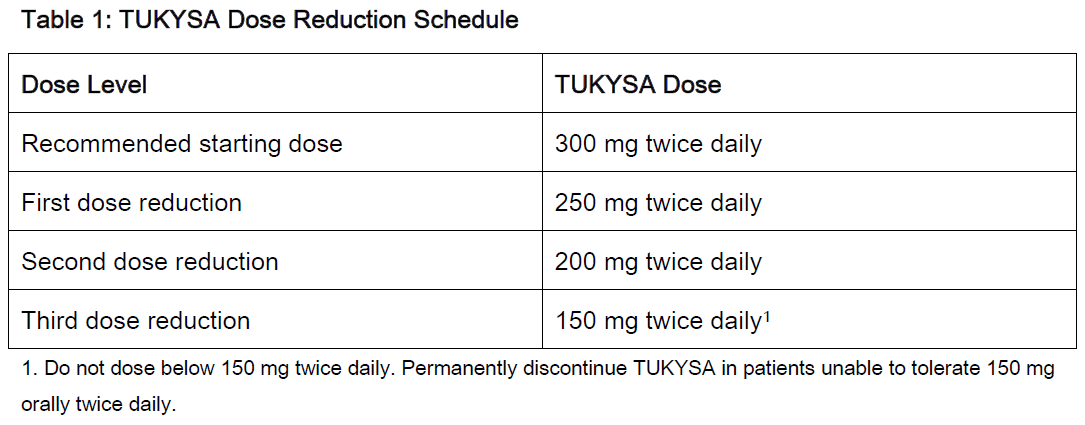 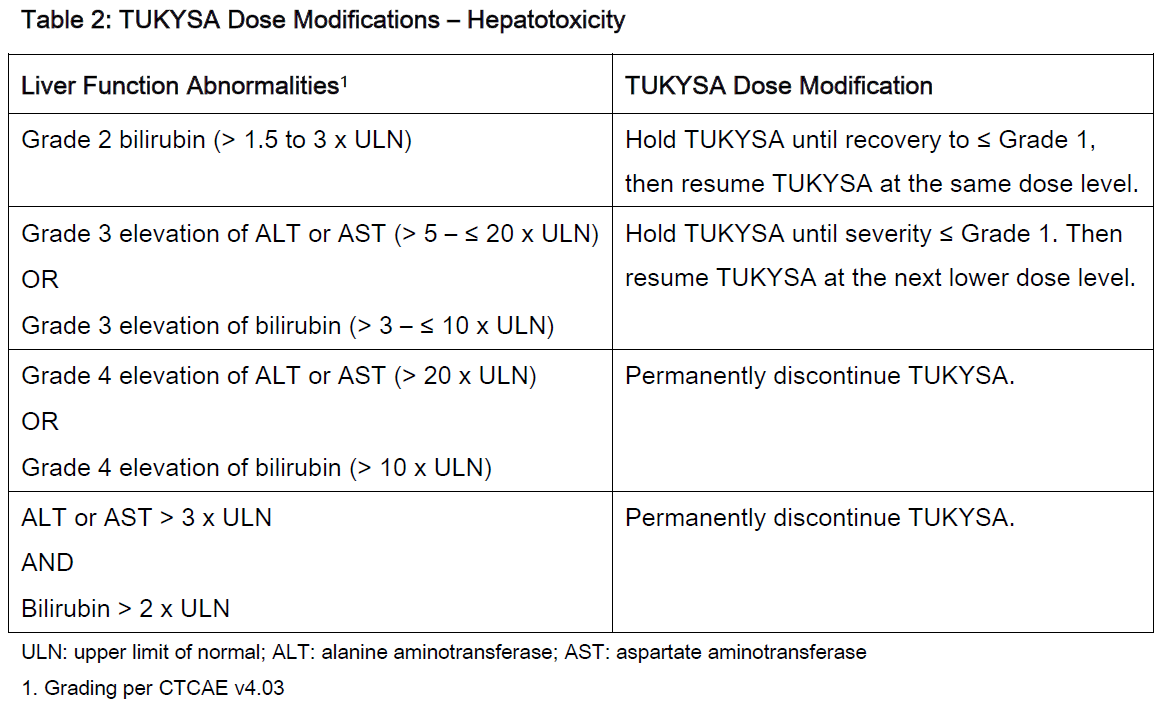 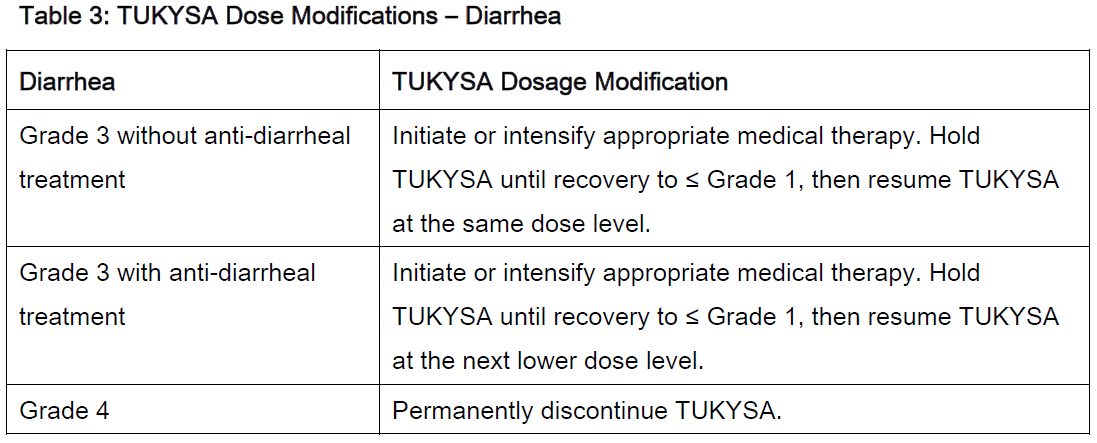 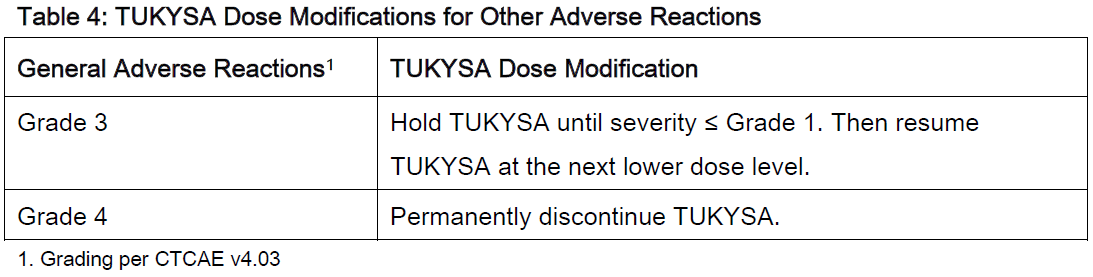 Dosage Modifications for Severe Hepatic Impairment For patients with severe hepatic impairment (Child-Pugh C), reduce the recommended dosage to 200 mg orally twice daily \[ _see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. Dosage Modifications for Concomitant Use with Strong CYP2C8 Inhibitors Avoid concomitant use of strong CYP2C8 inhibitors with TUKYSA. If concomitant use with a strong CYP2C8 inhibitor cannot be avoided, reduce the recommended dosage to 100 mg orally twice daily. After discontinuation of the strong CYP2C8 inhibitor for 3 elimination half-lives, resume the TUKYSA dose that was taken prior to initiating the inhibitor \[ _see Drug Interactions (7.2), Clinical Pharmacology (12.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
ORAL
Medical Information
**1 INDICATIONS AND USAGE** TUKYSA is indicated in combination with trastuzumab and capecitabine for treatment of patients with locally advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting. TUKYSA is indicated in combination with trastuzumab for the treatment of patients with RAS wild-type, HER2- positive unresectable or metastatic colorectal cancer that has progressed following treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
**4 CONTRAINDICATIONS** Hypersensitivity to the active substance or to any of the excipients contained in TUKYSA.
Pending
xpending
Manufacturer Information
MSD PHARMA (SINGAPORE) PTE. LTD.
Corden Pharma GmbH (Finished Tablets)
Hovione FarmaCiencia S.A (SDD intermediate)
Merck Sharp & Dohme B.V.
Active Ingredients
Documents
Package Inserts
Tukysa Tablets PI.pdf
Approved: March 27, 2023
