Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**Dosage** When Levemir® is used in combination with oral antidiabetic medicinal products or when added to GLP-1 receptor agonists, it is recommended to use Levemir® once daily, initially at a dose of 0.1–0.2 units/kg, or of 10 units **in adult patients**. The dose of Levemir® should be titrated based on the individual patient’s needs. When a GLP-1 receptor agonist is added to Levemir®, it is recommended to reduce the dose of Levemir® by 20% to minimise the risk of hypoglycaemia. Subsequently, dosage should be adjusted individually. For individual dose adjustments, the following two titration guidelines are recommended **for adults:** 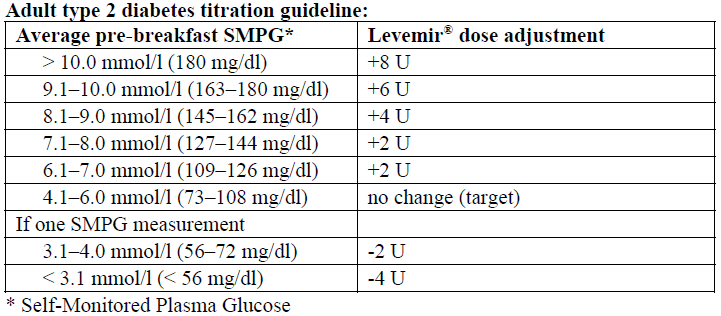 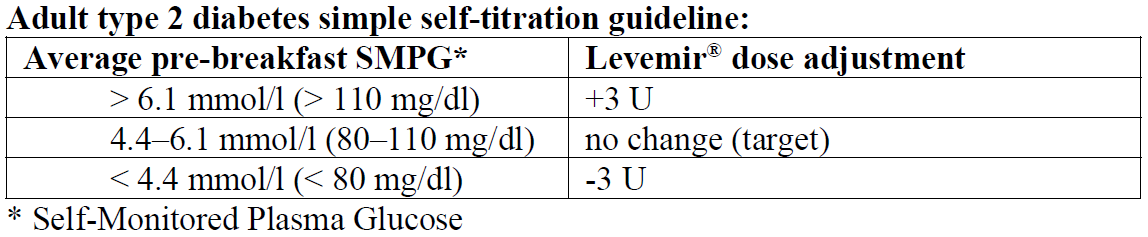 When Levemir® is used as part of a basal-bolus insulin regimen, Levemir® should be administered once or twice daily depending on the patient’s needs. The dose of Levemir® should be adjusted individually. For patients who require twice-daily dosing to optimise blood glucose control, the evening dose can be administered in the evening or at bedtime. Adjustment of dose may be necessary if patients undertake increased physical activity, change their usual diet or during concomitant illness. When adjusting dose in order to improve glucose control, patients should be advised to be aware of signs of hypoglycaemia. **Special populations** As with all insulin products, in elderly patients and patients with renal or hepatic impairment, glucose monitoring should be intensified and the Levemir® dosage adjusted on an individual basis. _Paediatric population_ The efficacy and safety of Levemir® were demonstrated in adolescents and children aged 2 years and above in studies up to 12 months (see _Pharmacodynamic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In children and adolescents, glucose monitoring should be intensified and the Levemir® dose adjusted on an individual basis. Levemir® has not been studied in children below the age of 2 years. **Transfer from other insulin products** Transfer to Levemir® from intermediate or long-acting insulin products may require adjustment of dose and timing of administration (see _Special warnings and precautions for use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). As with all insulin products, close glucose monitoring is recommended during the transfer and in the initial weeks thereafter. Concomitant antidiabetic treatment may need to be adjusted (dose and/or timing of oral antidiabetic medicines or concurrent short-acting insulin products). **Method of administration** Levemir® is for subcutaneous administration **only**. Levemir® must not be administered intravenously, as it may result in severe hypoglycaemia. Intramuscular administration should also be avoided. Levemir® is not to be used in insulin infusion pumps. Levemir® is administered subcutaneously by injection in the abdominal wall, the thigh, the upper arm, the deltoid region or the gluteal region. Injection sites should always be rotated within the same region in order to reduce the risk of lipodystrophy and cutaneous amyloidosis (see _Special warnings and precautions for use_ and _Undesirable effects_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). As with all insulin products, the duration of action will vary according to the dose, injection site, blood flow, temperature and level of physical activity. Levemir® Penfill® is designed to be used with Novo Nordisk insulin delivery systems and NovoFine® or NovoTwist® needles.
SUBCUTANEOUS
Medical Information
**Therapeutic indications** Treatment of diabetes mellitus in adults, adolescents and children aged 2 years and above.
**Contraindications** Hypersensitivity to the active substance or to any of the excipients (see _List of excipients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
A10AE05
insulin detemir
Manufacturer Information
NOVO NORDISK PHARMA (SINGAPORE) PTE LTD
Novo Nordisk A/S (Bagsværd) (Bulk Production and Primary Packager)
Novo Nordisk Produção Farmacêutica do Brasil Ltda.
Novo Nordisk Production SAS
Active Ingredients
Documents
Package Inserts
Levemir Penfill PI.pdf
Approved: February 9, 2022
