Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**DOSAGE AND ADMINISTRATION** **Recommended Dosage Regimen** Instruct patients to take VENCLEXTA tablets with a meal and water at approximately the same time each day. VENCLEXTA tablets should be swallowed whole and not chewed, crushed, or broken prior to swallowing. The 10, 50, and 100 mg strengths are interchangeable at equivalent doses (e.g., patients can take 2 x 50 mg tablets or 10 x 10 mg tablets instead of 1 x 100 mg VENCLEXTA tablet as needed) \[see **Pharmacokinetics** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. Chronic Lymphocytic Leukemia _VENCLEXTA Dose Ramp-Up Schedule_ The starting dose of VENCLEXTA is 20 mg once daily for 7 days. The VENCLEXTA dose must be administered according to a weekly ramp-up schedule to the daily dose of 400 mg over a period of 5 weeks as shown in Table 1. The 5-week ramp-up dosing schedule is designed to gradually reduce tumor burden (debulk) and decrease the risk of tumor lysis syndrome (TLS). 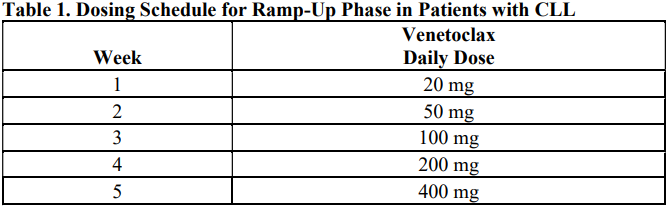 _VENCLEXTA in Combination with Obinutuzumab_ VENCLEXTA should be given for a total of 12 cycles: 6 cycles in combination with obinutuzumab, followed by 6 cycles of VENCLEXTA as a single agent. On Cycle 1 Day 1, start obinutuzumab administration at 100 mg, followed by 900 mg, which may be administered on Day 1 or Day 2. Administer 1000 mg on Days 8 and 15 of Cycle 1, and on Day 1 of five subsequent cycles (total of 6 cycles, 28 days each). On Cycle 1 Day 22, start VENCLEXTA according to the ramp-up schedule (see Table 1), continuing through Cycle 2 Day 28. After completing the ramp-up schedule, patients should continue VENCLEXTA 400 mg once daily from Cycle 3 Day 1 of obinutuzumab to the end of Cycle 12. _VENCLEXTA in Combination with Rituximab_ Start rituximab administration after the patient has completed the ramp-up schedule with VENCLEXTA (see Table 1) and has received the 400 mg dose of VENCLEXTA for 7 days. Administer rituximab on Day 1 of each 28-day cycle for 6 cycles, with rituximab dosed at 375 mg/m2 intravenously for Cycle 1 and 500 mg/m2 intravenously for Cycles 2–6. Patients should continue VENCLEXTA 400 mg once daily for 24 months from Cycle 1 Day 1 of rituximab. _VENCLEXTA as Monotherapy_ The recommended dose of VENCLEXTA is 400 mg once daily after the patient has completed the ramp-up schedule. VENCLEXTA should be taken orally once daily until disease progression or unacceptable toxicity is observed. Acute Myeloid Leukemia The dose of VENCLEXTA depends upon the combination agent. The VENCLEXTA dosing schedule (including ramp up) is shown in Table 2.  Initiate the hypomethylating agent or low-dose cytarabine on Cycle 1 Day 1. Azacitidine should be administered at 75 mg/m2 either intravenously or subcutaneously on Days 1–7 of each 28-day cycle beginning on Cycle 1 Day 1. Decitabine should be administered at 20 mg/m2 intravenously on Days 1–5 of each 28-day cycle beginning on Cycle 1 Day 1. Cytarabine should be administered at a dose of 20 mg/m2 subcutaneously once daily on Days 1–10 of each 28-day cycle beginning on Cycle 1 Day 1. Interrupt VENCLEXTA dosing as needed for management of hematologic toxicities and blood count recovery \[see **DOSE MODIFICATIONS BASED ON TOXICITIES**\]. Refer to the azacitidine, decitabine, or low-dose cytarabine prescribing information for additional information. VENCLEXTA, in combination with a hypomethylating agent or low-dose cytarabine, should be continued until disease progression or unacceptable toxicity is observed. **Missed Dose** If the patient misses a dose of VENCLEXTA within 8 hours of the time it is usually taken, the patient should take the missed dose as soon as possible and resume the normal daily dosing schedule. If a patient misses a dose by more than 8 hours, the patient should not take the missed dose and should resume the usual dosing schedule the next day. If the patient vomits following dosing, no additional dose should be taken that day. The next prescribed dose should be taken at the usual time. **Risk Assessment and Prophylaxis for Tumor Lysis Syndrome** Patients treated with VENCLEXTA may develop TLS. Refer to the appropriate section below for specific details on management. Assess patient-specific factors for level of risk of TLS and provide prophylactic hydration and anti-hyperuricemics to patients prior to first dose of VENCLEXTA to reduce risk of TLS. Chronic Lymphocytic Leukemia VENCLEXTA can cause rapid reduction in tumor, and thus poses a risk for TLS in the initial 5-week ramp-up phase. Changes in blood chemistries consistent with TLS that require prompt management can occur as early as 6 to 8 hours following the first dose of VENCLEXTA and at each dose increase. The risk of TLS is a continuum based on multiple factors, including comorbidities, particularly reduced renal function (creatinine clearance \[CrCl\] <80mL/min), and tumor burden. Splenomegaly may contribute to the overall TLS risk. The risk may decrease as tumor burden decreases with VENCLEXTA treatment \[see **WARNINGS AND PRECAUTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. Perform tumor burden assessments, including radiographic evaluation (e.g., CT scan). Assess blood chemistry (potassium, uric acid, phosphorus, calcium, and creatinine) in all patients and correct pre-existing abnormalities prior to initiation of treatment with VENCLEXTA. **Prophylaxis for Tumor Lysis Syndrome** Chronic Lymphocytic Leukemia Table 3 below describes the recommended TLS prophylaxis and monitoring during VENCLEXTA treatment based on tumor burden determination from clinical trial data. In addition, consider all patient comorbidities for risk-appropriate prophylaxis and monitoring, either outpatient or in hospital. 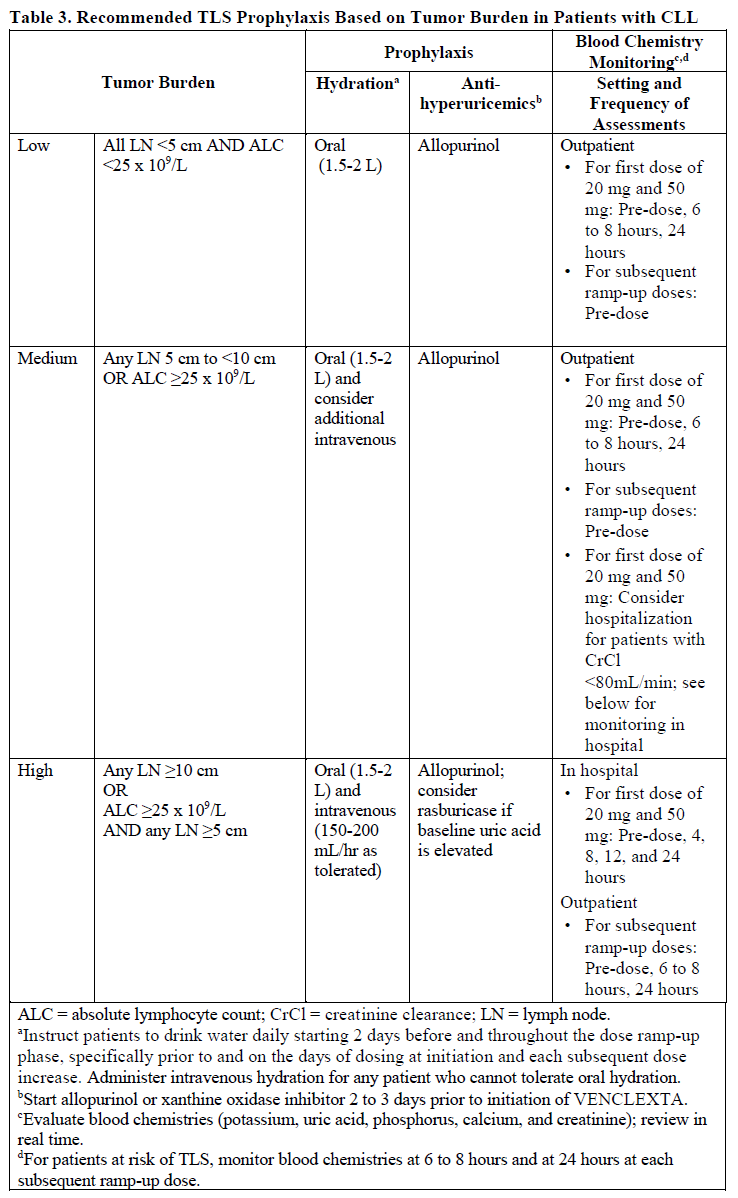 Acute Myeloid Leukemia The VENCLEXTA daily dose ramp-up is 3 days with azacitidine or decitabine, or 4 days with low-dose cytarabine (see Table 2). Follow prophylaxis measures listed below: - All patients should have white blood cell count <25 × 109/L prior to initiation of VENCLEXTA, and cytoreduction prior to treatment may be required. - All patients should be adequately hydrated and receive anti-hyperuricemic agents prior to initiation of first dose of VENCLEXTA and during ramp-up phase. - Assess blood chemistry (potassium, uric acid, phosphorus, calcium, and creatinine) and correct pre-existing abnormalities prior to initiation of treatment with VENCLEXTA. - Monitor blood chemistries for TLS at pre-dose, 6 to 8 hours after each new dose during ramp-up, and 24 hours after reaching final dose. - For patients with risk factors for TLS (e.g., circulating blasts, high burden of leukemia involvement in bone marrow, elevated pretreatment lactate dehydrogenase \[LDH\] levels, or reduced renal function), additional measures should be considered, including increased laboratory monitoring and reduced VENCLEXTA starting dose. **Dose Modifications Based on Toxicities** Chronic Lymphocytic Leukemia Dosing interruption and/or dose reduction for toxicities may be required. See Table 4 and Table 5 for recommended dose modifications for toxicities related to VENCLEXTA. For patients who have had a dosing interruption greater than 1 week during the first 5 weeks of ramp-up phase or greater than 2 weeks after completing the ramp-up phase, reassess for risk of TLS to determine if reinitiation with a reduced dose is necessary (e.g., all or some levels of dose ramp-up schedule) \[see **DOSAGE AND ADMINISTRATION**\]. 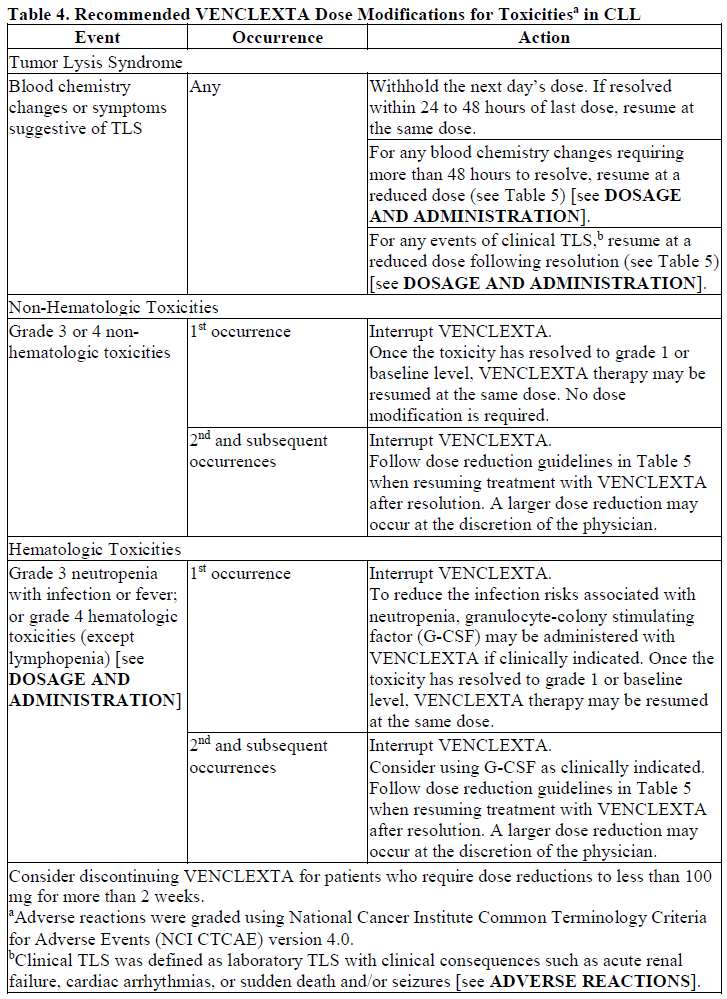 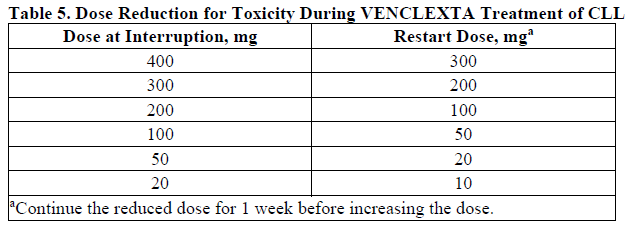 Acute Myeloid Leukemia _Dose modification for other toxicities_ Monitor blood counts frequently through resolution of cytopenias. Dose modification and interruptions for cytopenias are dependent on remission status. Dose modifications of VENCLEXTA for adverse reactions are provided in Table 6 \[see **WARNINGS AND PRECAUTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. 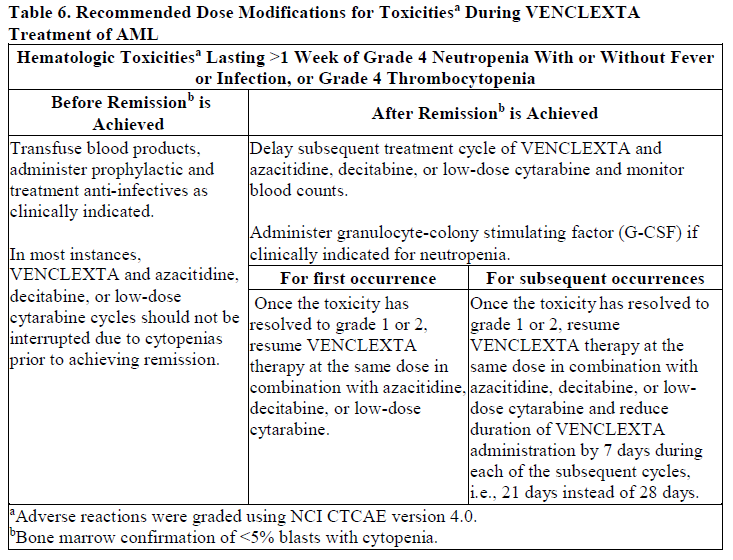 **Dose Modifications for Use with CYP3A Inhibitors** Concomitant use of VENCLEXTA with strong or moderate CYP3A inhibitors increases venetoclax exposure (i.e., Cmax and AUC) and may increase the risk for TLS at initiation and during ramp-up phase. In patients with CLL, concomitant use of VENCLEXTA with strong CYP3A inhibitors is contraindicated at initiation and during ramp-up phase \[see **CONTRAINDICATIONS**\]. In all patients, if a CYP3A inhibitor must be used, follow the recommendations for managing drug-drug interactions summarized in Table 7. Monitor patients more closely for signs of toxicities \[see **DOSAGE AND ADMINISTRATION**\]. Resume the VENCLEXTA dose that was used prior to initiating the CYP3A inhibitor 2 to 3 days after discontinuation of the inhibitor \[see **DOSAGE AND ADMINISTRATION** and **DRUG INTERACTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. 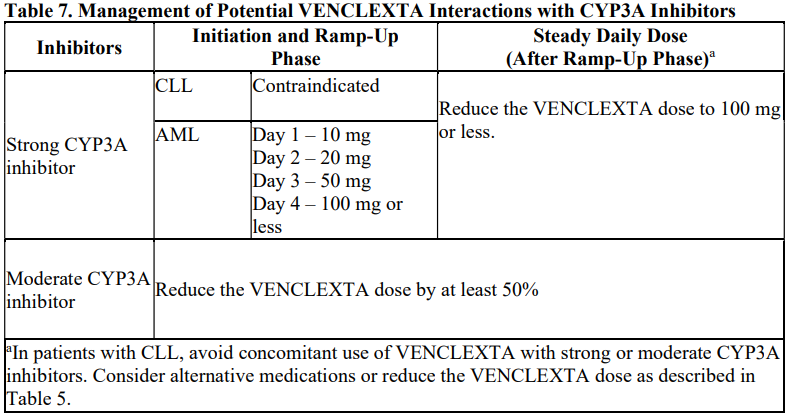
ORAL
Medical Information
**INDICATIONS** **Chronic Lymphocytic Leukemia** VENCLEXTA is indicated, in combination with rituximab or as monotherapy, for the treatment of patients with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy. VENCLEXTA is indicated, in combination with obinutuzumab, for the treatment of patients with previously untreated CLL. **Acute Myeloid Leukemia** VENCLEXTA is indicated, in combination with a hypomethylating agent or in combination with low-dose cytarabine, for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy \[see **CLINICAL STUDIES/USE IN SPECIFIC POPULATIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
**CONTRAINDICATIONS** In patients with CLL, concomitant use of VENCLEXTA with strong CYP3A inhibitors is contraindicated at initiation and during ramp-up phase \[see **DOSAGE AND ADMINISTRATION** and **DRUG INTERACTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
L01XX52
venetoclax
Manufacturer Information
ABBVIE PTE. LTD.
AbbVie Deutschland GmbH & Co. KG (Extrudate)
AbbVie Ireland NL B.V.
AbbVie Inc. (Primary & Secondary Packager)
