Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** _Route of administration_ Oral use The tablet should be swallowed with a sufficient amount of fluid (e.g. one glass of water). The tablet can be taken with or without food. _**Adults**_ **Herpes zoster infections** _Treatment of zoster-associated pain:_ - 1000 mg of valaciclovir 3 times daily for 7 days. Treatment should be initiated as soon as possible after the beginning of the infection, within 72 hours of the appearance of skin lesions. **Herpes simplex infections** _Treatment of genital herpes simplex infections in immunocompetent patients:_ - 500 mg twice daily for 10 days for the initial episode. - 1000 mg per day in one or two divided doses for 5 days for recurrent episodes. Treatment should be initiated as soon as possible in the course of infection, preferably at the prodromal stage or when lesions begin to appear. _Suppression of recurrent genital herpes simplex infections:_ - 500 mg per day in one or two divided doses (Better results have been obtained by dividing the daily dose into two, i.e. by administering 250 mg twice daily, when the administration of a single 500 mg dose per day failed, or if the recurrences were frequent or very symptomatic). For this indication, the need for treatment must be re-evaluated after 6 to 12 months. _**Adults and adolescents aged 12 years and above**_ _**Elderly**_ Dosage modification is not required unless renal function is significantly impaired (see Renal impairment, below). Adequate hydration must be maintained. 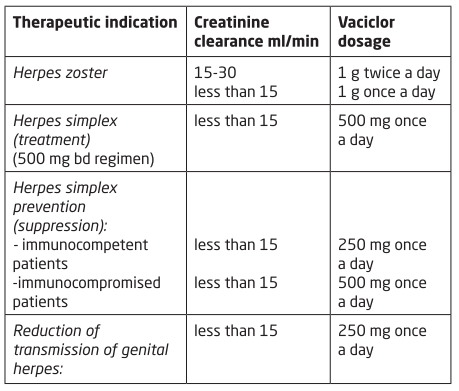 Patients on haemodialysis should receive the same dose as patients with creatinine clearance <10 ml/min. On dialysis days, the dose should be given after dialysis. In patients on haemodialysis, the dose must be administered after the haemodialysis has been performed. _**Hepatic impairment**_ Dose modification is not required in patients with mild or moderate cirrhosis (hepatic synthetic function maintained). Pharmacokinetic data in patients with advanced cirrhosis (impaired hepatic synthetic function and evidence of portal-systemic shunting) do not indicate the need for dosage adjustment. However, clinical experience is limited. _**Children below the age of 12 years**_ Valaciclovir is not recommended for use in children below the age of 12 years due to insufficient data on safety and efficacy.
ORAL
Medical Information
**4.1 Therapeutic indications** Vaciclor is indicated for the treatment of herpes zoster (shingles). Vaciclor accelerates the resolution of pain; it reduces the duration of and the proportion of patients with zoster-associated pain, which includes acute and post-herpetic neuralgia. Vaciclor is indicated for the treatment of herpes simplex infection of the skin and mucous membranes, including initial and recurrent genital herpes. Vaciclor can prevent lesion development when taken at the first signs and symptoms of an HSV recurrence. Vaciclor is indicated for the prevention (suppression) of recurrent herpes simplex infections of the skin and mucous membranes, including genital herpes. Consideration should be given to official guidance on the appropriate use of antiviral agents.
**4.3 Contraindications** Hypersensitivity to valaciclovir, aciclovir or to any of the excipients.
J05AB11
valaciclovir
Manufacturer Information
TEVA PHARMACEUTICAL INVESTMENTS SINGAPORE PTE. LTD.
BALKANPHARMA - DUPNITSA AD
Active Ingredients
Documents
Package Inserts
Vaciclovir Tablet 500mg_PI.pdf
Approved: May 8, 2023
