Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SPRAY, METERED
**4.2 Posology and method of administration** Treatment should be initiated by and remain under the supervision of a physician experienced in the management of opioid therapy in cancer patients. Physicians should keep in mind the potential for abuse of fentanyl. Posology PecFent should be titrated to an “effective” dose that provides adequate analgesia and minimises adverse reactions without causing undue (or intolerable) adverse reactions, for two consecutively treated episodes of BTP. The efficacy of a given dose should be assessed over the ensuing 30-minute period. Patients should be carefully monitored until an effective dose is reached. PecFent is available in two strengths: 100 micrograms/spray and 400 micrograms/spray. One dose of PecFent may include administration of 1 spray (100 microgram or 400 microgram doses) or 2 sprays (200 microgram or 800 microgram doses) of the same strength (either 100 microgram or 400 microgram strength). Patients should not use more than 4 doses per day. Patients should wait at least 4 hours after a dose before treating another BTP episode with PecFent. PecFent can deliver 100, 200, 400 and 800 microgram doses as follows: 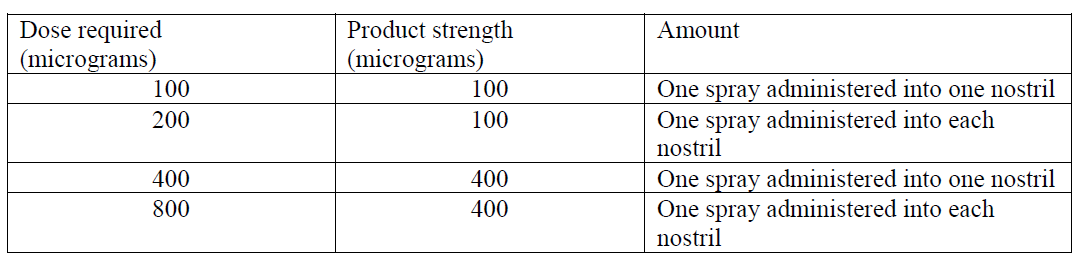 _Initial dose_ - The initial dose of PecFent to treat episodes of BTP is always 100 micrograms (one spray), even in patients switching from other fentanyl-containing products for their BTP. - Patients must wait at least 4 hours before treating another episode of BTP with PecFent. _Method of titration_ - Patients should be prescribed an initial titration supply of one bottle (8 sprays) of PecFent 100 micrograms/spray. - Patients whose initial dose is 100 micrograms and who need to titrate to a higher dose due to a lack of effect can be instructed to use two 100 microgram sprays (one in each nostril) for their next BTP episode. If this dose is not successful, the patient may be prescribed a bottle of PecFent 400 micrograms/spray and instructed to change to one 400 microgram spray for their next episode of pain. If this dose is not successful, the patient may be instructed to increase to two 400 microgram sprays (one in each nostril). - From treatment initiation, patients should be closely followed and the dose titrated until an effective dose is reached and confirmed for two consecutively treated episodes of BTP. _Titration in patients switching between immediate-release fentanyl-containing products_ Substantial differences may exist in the pharmacokinetic profile of immediate-release fentanyl medicinal products, which result in clinically important differences in the rate and extent of absorption of fentanyl. Therefore, when switching between fentanyl-containing medicinal products indicated for treatment of breakthrough pain, including intranasal formulations, it is essential that patients are again titrated with the new medicinal product, and not switched on a dose-for-dose (microgram-for-microgram) basis. _Maintenance therapy_ Once an effective dose has been established during titration, patients should continue to take this dose up to a maximum of 4 doses per day. _Dose readjustment_ Generally, the maintenance dose of PecFent should be increased only where the current dose fails to adequately treat the BTP for several consecutive episodes. A review of the dose of the background opioid therapy may be required if patients consistently present with more than four BTP episodes per 24 hours. In absence of adequate pain control, the possibility of hyperalgesia, tolerance and progression of underlying disease should be considered (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If adverse reactions are intolerable or persistent, the dose should be reduced or treatment with PecFent replaced by another analgesic. _Discontinuation of therapy_ PecFent should be discontinued immediately if the patient no longer experiences breakthrough pain episodes. The treatment for persistent background pain should be kept as prescribed. If discontinuation of all opioid therapy is required, the patient must be closely followed by the doctor as gradual downward opioid titration therapy is necessary in order to avoid the possibility of abrupt withdrawal effects. _Special populations_ _Elderly (older than 65 years)_ In the PecFent clinical trial programme, 104 (26.1%) of patients were over 60 years of age, 67 (16.8%) over 65 years and 15 (3.8%) over 75 years. There was no indication that older patients tended to titrate to lower doses or experience more adverse reactions. Nevertheless, in view of the importance of renal and hepatic function in the metabolism and clearance of fentanyl, additional care should be exercised in the use of PecFent in the elderly. No data on the pharmacokinetics of PecFent in elderly patients are available. _Hepatic or renal impairment_ PecFent should be administered with caution to patients with moderate or severe hepatic or renal impairment (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ The safety and efficacy of PecFent in children and adolescents aged below 18 years have not yet been established. No data are available. Method of administration PecFent is for nasal use only. The bottle should be removed from the child-resistant container immediately prior to use and the protective cap removed. The bottle must be primed before first use by holding upright and simply pressing and releasing the finger grips either side of the nozzle until a green bar appears in the counting window (should occur after four sprays). 8 spray bottle: If the product has not been used for 5 days, it should be re-primed by spraying once. The patient should be advised to write the date of first use in the space provided on the label of the child-resistant container. To administer PecFent the nozzle is placed a short distance (about 1 cm) into the nostril and pointed slightly towards the bridge of the nose. A spray is then administered by pressing and releasing the finger grips either side of the nozzle. An audible click will be heard and the number displayed on the counter will advance by one. Patients must be advised that they may not feel the spray being administered, and that they should, therefore, rely on the audible click and the number on the counter advancing to confirm that a spray has been delivered. The PecFent spray droplets form a gel in the nose. Patients should be advised not to blow their nose immediately after PecFent administration. The protective cap should be replaced after each use and the bottle returned to the child-resistant container for safe storage.
NASAL
Medical Information
**4.1 Therapeutic indications** PecFent is indicated for the management of breakthrough pain (BTP) in adults who are already receiving maintenance opioid therapy for chronic cancer pain. Breakthrough pain is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent pain. Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Patients without maintenance opioid therapy as there is an increased risk of respiratory depression. Severe respiratory depression or severe obstructive lung conditions. Treatment of acute pain other than breakthrough pain. Patients being treated with medicinal products containing sodium oxybate.
N02AB03
fentanyl
Manufacturer Information
A. MENARINI SINGAPORE PTE. LTD.
L. MOLTENI & C. DEI. F.LLI ALITTI SOCIETA’ DI ESERCIZIO S.P.A.
Active Ingredients
Documents
Package Inserts
Pecfent Nasal Spray Solution PI.pdf
Approved: March 9, 2023
