Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER, FOR SOLUTION
**4.2 Posology and method of administration** This medicinal product should only be used by experienced neurosurgeons conversant with surgery of malignant gliomas and in-depth knowledge of functional brain anatomy who have completed a training course in fluorescence-guided surgery. Posology The recommended dose is 20 mg 5-ALA HCl per kilogram body weight. The total number of bottles needed to achieve the intended dose for the individual patient can be determined according to the equation below (rounded up to the nearest whole bottle):  The administration volume needed to achieve the intended dose for the individual patient can be calculated according to the equation below: 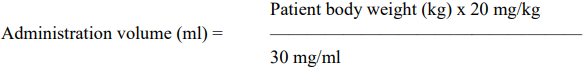 _Renal or hepatic impairment_ No trials have been performed in patients with clinically relevant hepatic or renal impairment. Therefore, this medicinal product should be used with caution in such patients. _Elderly_ There are no special instructions for use in elderly patients with regular organ function. _Paediatric population_ The safety and efficacy of Gliolan in children and adolescents aged 0 to 18 years have not yet been established. No data are available. Method of administration The solution should be administered orally three hours (range 2–4 hours) before anaesthesia. Use of 5-ALA under conditions other than the ones used in the clinical trials entail an undetermined risk. If the surgery is postponed by more than 12 hours, surgery should be re-scheduled for the next day or later. Another dose of this medicine can be taken 2 – 4 hours before anaesthesia. _Precautions to be taken before handling or administering the medicinal product_ For instructions on reconstitution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
ORAL
Medical Information
**4.1 Therapeutic indications** Gliolan is indicated in adults for visualisation of malignant tissue during surgery for malignant glioma (WHO grade III and IV).
**4.3 Contraindications** - Hypersensitivity to the active substance or porphyrins. - Acute or chronic types of porphyria. - Pregnancy (see sections 4.6 and 5.3 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01XD04
aminolevulinic acid
Manufacturer Information
LINK HEALTHCARE SINGAPORE PTE. LTD.
LyoContract GmBH
Active Ingredients
Aminolevulinic acid 23.4mg/ml equivalent aminolevulinic acid hydrochloride
30mg/ml
