Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Posology _Dosing Considerations_ - Treatment with Xospata should be initiated and supervised by a physician experienced in the use of anticancer therapies. - Prior to initiation of treatment with Xospata, patients must have confirmation of _FLT3_ mutation (internal tandem duplication \[ITD\] or tyrosine kinase domain \[TKD\]) using a validated test. - Assess blood counts and chemistries, including creatine phosphokinase, prior to the initiation of treatment with Xospata, once weekly for the first month, once every other week for the second month, and monthly for the duration of therapy. - Perform electrocardiogram (ECG) prior to initiation of treatment with Xospata, on days 8 and 15 of the first month, prior to the start of the next two months of treatment, and then as clinically indicated. _Recommended Dose and Dosage Adjustment_ The recommended dose of Xospata is 120 mg (three 40 mg tablets) orally once daily with or without food (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Treatment should continue as long as clinical benefit is observed or until unacceptable toxicity occurs. Clinical response can be delayed (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In the absence of disease progression or unacceptable toxicity, treatment for a minimum of 6 months is recommended to allow time for a clinical response. No dose adjustment is required in geriatric patients (≥ 65 years of age) (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No dose adjustment is required in patients with mild or moderate renal impairment (creatinine clearance \[CLCr\] ≥ 30 mL/min). Clinical experience in patients with severe renal impairment (CLCr < 30 mL/min) is limited (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No dose adjustment is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No data are available in patients with severe hepatic impairment (Child-Pugh Class C) (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 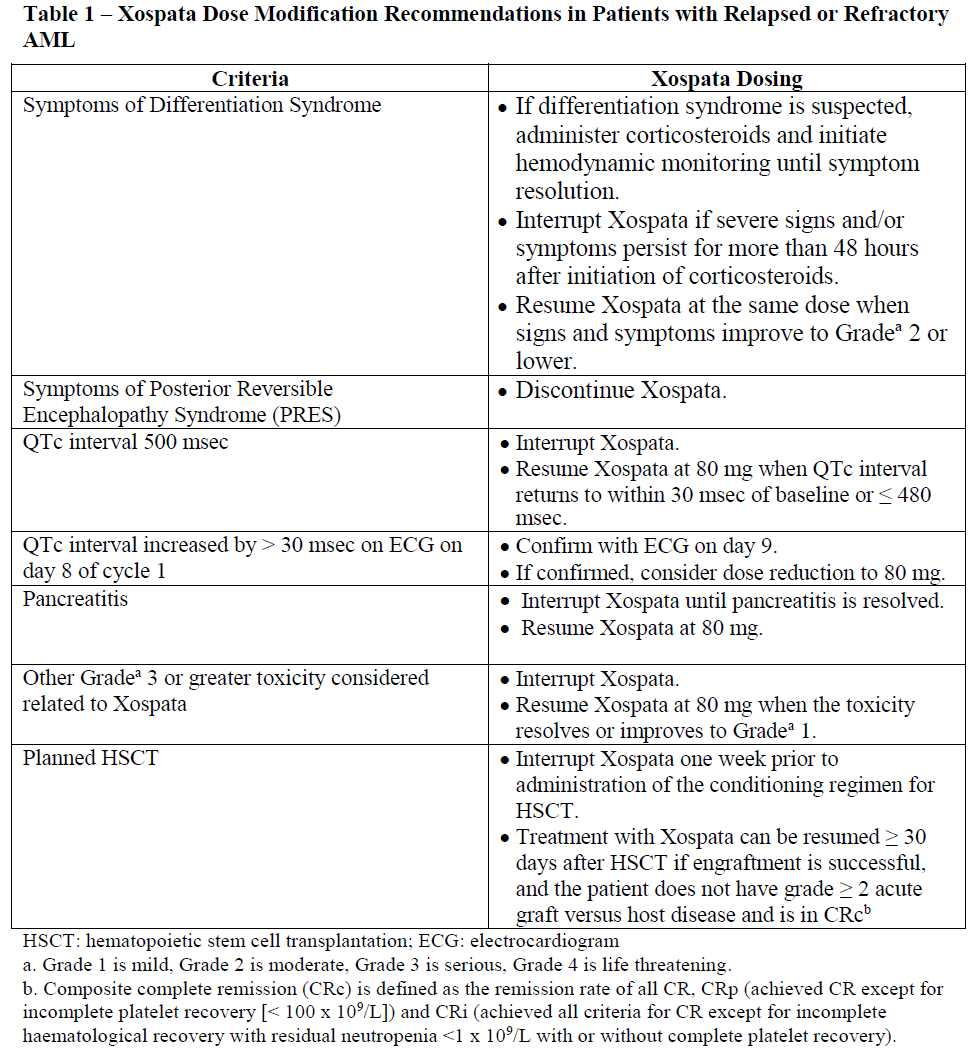 _Paediatric population (< 18 years of age)_ The safety and efficacy of Xospata in children has not been established. Animal studies have demonstrated toxicity in juvenile rats (see section 5.3 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Geriatric population (≥ 65 years of age)_ No overall differences in efficacy or safety were observed between patients 65 years or older and younger patients. Method of administration Administer Xospata tablets orally about the same time each day. Do not break or crush tablets. _Missed Dose_ Xospata should be administered at about the same time each day. If a dose is missed or not taken at the usual time, the dose should be administered as soon as possible on the same day, and patients should return to the normal schedule the following day. Do not administer 2 doses within 12 hours. If vomiting occurs after dosing, patients should not take another dose but should return to the normal schedule the following day.
ORAL
Medical Information
**4.1 Therapeutic indications** Xospata® (gilteritinib tablets) is indicated for: - the treatment of adult patients who have relapsed or refractory acute myeloid leukemia (AML) with a FMS-like tyrosine kinase 3 ( _FLT3_) mutation. A validated test is required to confirm the FLT3 mutation status of AML.
**4.3 Contraindications** Hypersensitivity to the active substance(s) or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01EX13
gilteritinib
Manufacturer Information
ASTELLAS PHARMA SINGAPORE PTE. LTD.
Astellas Pharma Inc. (Yaizu Technology Center)
Astellas Pharma Europe B.V. (Primary and Secondary Packager)
Active Ingredients
Documents
Package Inserts
Xospata Film-Coated Tablets PI.pdf
Approved: August 12, 2022
