Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER, FOR SUSPENSION
**Dosage and Administration** Pharmaceutical form: Powder for oral suspension. Dosage depends on the age, weight and renal function of the patient and the severity of the infection. Dosages are expressed throughout in terms of amoxicillin/clavulanate content except when doses are stated in terms of an individual component. To minimise potential gastrointestinal intolerance, administer at the start of a meal. The absorption of _AUGMENTIN_ is optimised when taken at the start of a meal. Treatment should not exceed 14 days without review. Therapy can be started parenterally and continued with an oral preparation. _AUGMENTIN_ bottle presentations for suspension may be supplied with a plastic dosing device. For preparation of the suspensions, see _Instructions for Use/Handling – please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The usual recommended daily dosage is: - 25/3.6 mg/kg/day in mild to moderate infections (upper respiratory tract infections e.g. recurrent tonsillitis, lower respiratory infections and skin and soft tissue infections). - 45/6.4 mg/kg/day for the treatment of more serious infections (upper respiratory tract infections e.g. otitis media and sinusitis, lower respiratory tract infections e.g. bronchopneumonia and urinary tract infections). No clinical data are available on doses above 45/6.4 mg/kg/day in children under 2 years. There are no clinical data for _AUGMENTIN_ suspension 228 mg/5 mL and 457 mg/5 mL to make dosage recommendations for children under 2 months old. The tables below give dosage guidance for children. _Children 2 years and over_  _Children aged 2 months to under 2 years_ Children under 2 years should be dosed according to body weight. 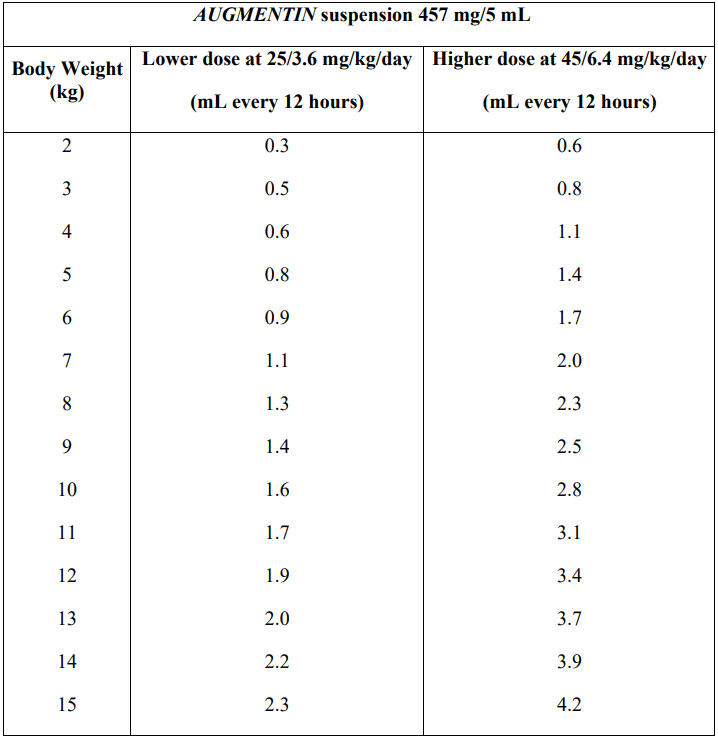 _Renal Impairment_ No adjustment in dose is required in patients with creatinine clearance greater than 30 mL/min. _AUGMENTIN_ suspension 228 mg/5 mL and 457 mg/5 mL are not recommended in patients with a creatinine clearance of less than 30 mL/min. _Hepatic Impairment_ Dose with caution; monitor hepatic function at regular intervals. There are insufficient data on which to base a dosage recommendation.
ORAL
Medical Information
**Indications** _AUGMENTIN_ should be used in accordance with local official antibiotic prescribing guidelines and local susceptibility data. _AUGMENTIN_ suspension (228 mg/5 mL and 457 mg/5 mL), for twice daily oral dosing, is indicated for short term treatment of bacterial infections at the following sites when amoxicillin resistant beta-lactamase producing strains are suspected as the cause. In other situations, amoxicillin alone should be considered. _Upper respiratory tract infections (including ENT)_ e.g. recurrent tonsillitis, sinusitis, otitis media. _Lower respiratory tract infections_ e.g. acute exacerbations of chronic bronchitis, lobar and bronchopneumonia. _Urinary tract infections_ e.g. cystitis, urethritis, pyelonephritis. _Skin and soft tissue infections_ e.g. cellulitis, animal bites. Susceptibility to _AUGMENTIN_ will vary with geography and time (see _Pharmacological Properties, Pharmacodynamics_ for further information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Local susceptibility data should be consulted where available, and microbiological sampling and susceptibility testing performed where necessary. Mixed infections caused by amoxicillin-susceptible organisms in conjunction with _AUGMENTIN_ susceptible beta-lactamase producing organisms may be treated with _AUGMENTIN_ suspension 228 mg/5 mL and 457 mg/5 mL. These infections should not require the addition of another antibiotic resistant to beta-lactamases.
**Contraindications** _AUGMENTIN_ is contraindicated - in patients with a history of hypersensitivity to beta-lactams, e.g. penicillins and cephalosporins. - in patients with a previous history of _AUGMENTIN_-associated jaundice/hepatic dysfunction.
J01CR02
amoxicillin and beta-lactamase inhibitor
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
SmithKline Beecham Limited trading as SmithKline Beecham Pharmaceuticals
Glaxo Wellcome Production
Active Ingredients
Documents
Package Inserts
Augmentin For Oral Solution PI.pdf
Approved: October 4, 2022
