Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** PREZISTA® must always be given with low dose ritonavir as a pharmacokinetic enhancer and in combination with other antiretroviral medicinal products. The prescribing information of ritonavir must therefore be consulted prior to initiation of therapy with PREZISTA®/rtv. After therapy with PREZISTA® has been initiated, patients should be advised not to alter the dosage or discontinue therapy without instruction of their physician. Method of administration: oral administration. PREZISTA® must be taken with food. The type of food does not affect the exposure to PREZISTA® (see _Pharmacokinetic Properties – Absorption_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Dosage – Adults** **_Treatment-Naïve Adult Patients_** The recommended oral dose of PREZISTA® tablets is 800 mg (one 800 mg tablet or two 400 mg tablets) taken with ritonavir 100 mg once daily and with food. _**Treatment-Experienced Adult Patients**_ 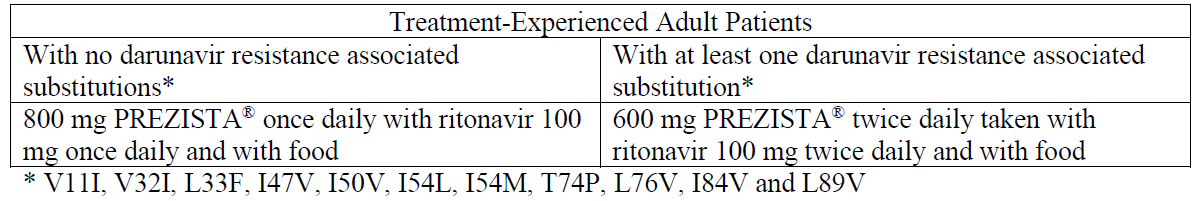 For antiretroviral treatment-experienced patients, HIV genotypic testing is recommended. However, when HIV genotypic testing is not feasible, the PREZISTA®/ritonavir 600/100 mg twice daily dosing is recommended. The type of food does not affect the exposure to darunavir. Ritonavir (100 mg) is used as a pharmacokinetic enhancer of darunavir (see _Interactions_ and _Pharmacokinetic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Dosage – Pediatrics** **_Antiretroviral treatment-experienced pediatric patients (6 to < 18 years of age)_** The recommended dose of PREZISTA®/rtv for pediatric patients (6 to < 18 years of age and weighing at least 20 kg) is based on body weight (see table below) and should not exceed the recommended adult dose (600/100 mg b.i.d.). PREZISTA® tablets should be taken with ritonavir twice daily and with food. The type of food does not affect exposure to darunavir. 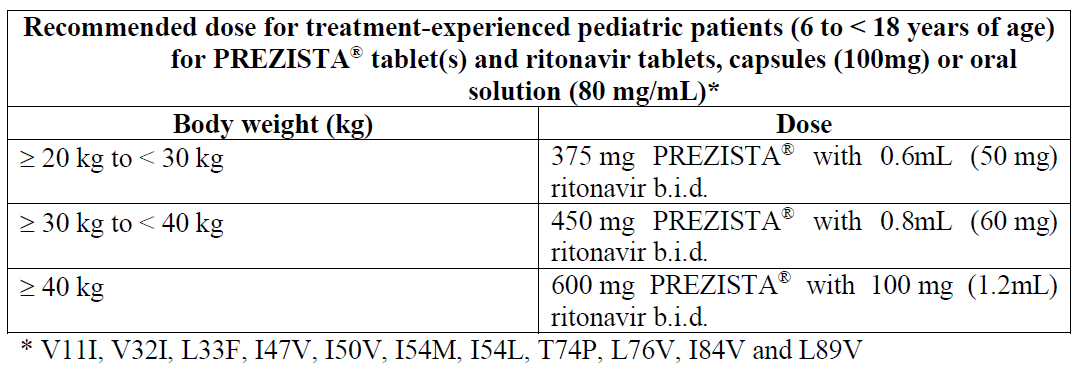 _**Antiretroviral treatment-experienced children less than 6 years of age and antiretroviral treatment-naïve pediatric patients**_ The safety and efficacy of PREZISTA®/rtv in antiretroviral treatment-experienced children aged 3 to less than 6 years and in antiretroviral treatment-naïve pediatric patients have not been established. PREZISTA®/rtv should not be used in children below 3 years of age (See _Warnings and Precautions_ and _Non-Clinical Information_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Pregnancy and postpartum**_ No dose adjustment is required for PREZISTA®/rtv during pregnancy and postpartum. Caution should be used in patients with concomitant medications which may further decrease darunavir exposure (see _Pregnancy, Breast-feeding and Fertility_ and _Pharmacokinetic Properties – Special Populations – Pregnancy and Postpartum_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Missed dose(s)** If using the once daily regimen: in case a dose of PREZISTA® and/or ritonavir was missed within 12 hours of the time it is usually taken, patients should be instructed to take the prescribed dose of PREZISTA® and ritonavir with food as soon as possible. If this was noticed later than 12 hours after the time it is usually taken, the missed dose should not be taken and the patient should resume the usual dosing schedule. If using the twice daily regimen: in case a dose of PREZISTA® and/or ritonavir was missed within 6 hours of the time it is usually taken, patients should be instructed to take the prescribed dose of PREZISTA® and ritonavir with food as soon as possible. If this was noticed later than 6 hours of the time it is usually taken, the missed dose should not be taken and the patient should resume the usual dosing schedule. **Special populations** **_Elderly (65 years of age and older)_** Limited information is available on the use of PREZISTA® in patients 65 and older. Therefore PREZISTA® should be used with caution in this age group (see _Warnings and Precautions_ and _Pharmacokinetic Properties – Elderly_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Renal impairment**_ No dose adjustment is required in patients with renal impairment (see _Warnings and Precautions_ and _Pharmacokinetic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Hepatic impairment**_ No dose adjustment is required in patients with mild or moderate hepatic impairment. There are no pharmacokinetic or safety data available for subjects with severe hepatic impairment, therefore, PREZISTA®/rtv must not be used in patients with severe hepatic impairment (see _Warnings and Precautions_ and _Pharmacokinetic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Administration** **Method of administration:** oral administration. PREZISTA® must be taken with food. The type of food does not affect the exposure to PREZISTA® (see _Pharmacokinetic Properties – Absorption_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**Indications** **Adult patients** PREZISTA®, in combination with 100 mg low dose ritonavir (PREZISTA®/rtv) and with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus (HIV) infection. **Pediatric patients** PREZISTA®, in combination with low dose ritonavir (PREZISTA®/rtv) and with other antiretroviral agents, is indicated for the treatment of HIV infection in treatment-experienced pediatric patients of 6 years and above and at least 20kg body weight. In treatment-experienced adult and pediatric patients, the following points should be considered when initiating therapy with PREZISTA®/rtv: - Treatment history and, when available, genotypic or phenotypic testing should guide the use of PREZISTA®/rtv.
**Contraindications** Hypersensitivity to darunavir or to any of the excipients. Darunavir and ritonavir are both inhibitors of the cytochrome P450 3A (CYP3A) isoform. PREZISTA®/rtv should not be co-administered with medicinal products that are highly dependent on CYP3A for clearance and for which increased plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). Examples include alfuzosin, astemizole, cisapride, colchicine (in patients with renal and/or hepatic impairment), dapoxetine, dronedarone, elbasvir/grazoprevir, the ergot alkaloids (e.g., ergotamine, dihydroergotamine, ergonovine and methylergonovine), ivabradine, lomitapide, lovastatin, lurasidone, midazolam (oral), naloxegol, pimozide, ranolazine, sildenafil (when used for treatment of pulmonary arterial hypertension), simvastatin, terfenadine, ticagrelor, and triazolam (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients taking PREZISTA® should not use products containing potent CYP3A inducers such as rifampin or St. John’s wort because co-administration may result in reduced plasma concentrations of darunavir. This may result in loss of therapeutic effect and development of resistance.
J05AE10
darunavir
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
JANSSEN-ORTHO LLC
Active Ingredients
Documents
Package Inserts
Prezista_PI.pdf
Approved: March 13, 2023
