Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**Dosage & Administration** The dosage should be adapted to the individual requirements and the patients should be kept under medical supervision during treatment. It is advisable not to exceed the recommended daily dose during either acute or maintenance treatment. If therapy does not produce a significant improvement or if the patient’s condition gets worse, medical advice must be sought in order to determine a new plan of treatment. The patient should be instructed that in the case of acute or rapidly worsening dyspnoea physician should be consulted immediately. The following dosages are recommended: **For ATROVENT® 20 mcg/puff Metered Dose Inhaler** _Maintenance treatment_ Adults and children > 6 years of age: 2 metered doses (puffs) 4 times daily. Since a requirement for increasing doses suggests that additional therapeutic modalities may be needed, a total daily dose of 12 puffs should generally not be exceeded. For acute exacerbations of chronic obstructive pulmonary disease treatment with ATROVENT® nebuliser solution may be indicated. Because of insufficient information in children ATROVENT® metered dose aerosol should only be used on medical advice and under the supervision of an adult. **For ATROVENT® 0.025% Nebuliser Solution** (20 drops = about 1 mL; 1 drop = 0.0125 mg ipratropium bromide anhydrous) _Maintenance treatment:_ Adults (including elderly) and adolescents over 12 years of age: 2.0 mL (40 drops = 0.5 mg ipratropium bromide anhydrous) 3 to 4 times daily Children 6 – 12 years: Because there is limited information in this age group, the following dose recommendation should be given under medical supervision: 1.0 mL (20 drops = 0.25 mg ipratropium bromide anhydrous) 3 to 4 times daily Children < 6 years of age: Because there is limited information in this age group the following dose recommendation should be given under medical supervision: 0.4 – 1.0 mL (8 – 20 drops = 0.1 – 0.25 mg ipratropium bromide anhydrous) 3 to 4 times daily _Acute attacks:_ Adults (including elderly) and adolescents > 12 years of age: 2.0 mL (40 drops = 0.5 mg ipratropium bromide anhydrous); repeated doses can be administered until the patient is stable. The time interval between the doses may be determined by the physician. ATROVENT® can be administered combined with an inhaled beta-agonist. Children 6 – 12 years of age: Because there is limited information in this age group, the following dose recommendation should be given under medical supervision: 1.0 mL (20 drops = 0.25 mg ipratropium bromide anhydrous); repeated doses can be administered until the patient is stable. The time interval between the doses may be determined by the physician. ATROVENT® can be administered combined with an inhaled beta-agonist. Children < 6 years of age: Because there is limited information in this age group the following dose recommendation should be given under medical supervision: 0.4 – 1.0 mL (8 – 20 drops = 0.1 – 0.25 mg ipratropium bromide anhydrous); repeated doses can be administered until the patient is stable. The time interval between the doses may be determined by the physician. ATROVENT® can be administered combined with an inhaled beta-agonist. Daily doses exceeding 2 mg ipratropium bromide anhydrous in adults and adolescents >12 years of age and 1 mg in children ≤ 12 years of age should be given under medical supervision. **Instructions for use** Please read the instructions for use carefully, to ensure correct administration. **For ATROVENT® 20 mcg/puff Metered Dose Inhaler** The correct administration is essential for successful therapy. - Before first time use: Depress the valve twice before the inhaler is used - Before each use the following rules should be observed: 1. Remove protective cap. 2. Breathe out deeply. 3. Hold the inhaler as shown in fig. 1, and close lips over the mouthpiece. The arrow and the base of the container should be pointing upwards 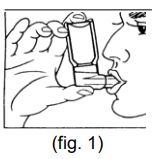 4. Breathe in as deeply as possible, pressing the base of the canister firmly at the same time, this releases one metered dose. Hold the breath for a few seconds, then remove the mouthpiece and breathe out. The same action should be repeated for a second inhalation. 5. Replace the protective cap after use. 6. After not using the inhaler for three days the valve has to be actuated once. The container is not transparent. It is therefore not possible to see when it is empty. The inhaler will deliver **200** puffs. When the labelled number of doses have been used (usually after 3 weeks when used as recommended), the canister may still appear to contain a small amount of fluid. The inhaler should, however, be replaced so that you can be certain that you are getting the right amount of your medicine in each actuation. Clean your mouthpiece at least once a week. It is important to keep the mouthpiece of your inhaler clean to ensure that medicine does not build up and block the spray. For cleaning, first take off the dust cap and remove the canister from the mouthpiece. Rinse warm water through the mouthpiece until no medication build-up and/or dirt is visible. 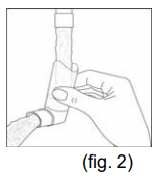 After cleaning shake out the mouthpiece and let it air-dry **without** using any heating system. Once the mouthpiece is dry, replace the canister and the dust cap. 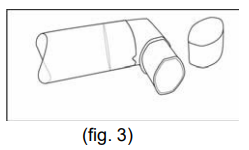 WARNING: The plastic mouthpiece has been specially designed for use with ATROVENT® metered dose inhaler to ensure that you always get the right amount of the medicine. The mouthpiece must never be used with any other metered dose inhaler nor must the ATROVENT® metered dose inhaler be used with any mouthpiece other than the one supplied with the product. The container is under pressure and should by no account be opened by force or exposed to temperatures above 50°C. **For ATROVENT® 0.025 % Nebuliser Solution** The recommended dose is to be diluted with physiological saline to a final volume of 3 – 4 mL and nebulised and inhaled until the solution is consumed. The solution should be rediluted each time before use; any residual diluted solution should be discarded. Dosage may be dependent upon the mode of inhalation and the quality of nebulisation. The duration of inhalation can be controlled by the dilution volume. ATROVENT® Nebuliser Solution can be administered using a range of commercially available nebulising devices. Where wall oxygen is available the solution is best administered at a flow rate of 6 – 8 litres per minute. ATROVENT® Nebuliser Solution is suitable for concurrent inhalation with the Nebuliser Solution of the secretomucolytics ambroxol hydrochloride and bromhexine hydrochloride, or fenoterol hydrobromide. ATROVENT® Nebuliser Solution and disodium cromoglycate Nebuliser Solution should not be administered simultaneously in the same nebuliser as precipitation may occur.
RESPIRATORY (INHALATION)
Medical Information
**Indications** For ATROVENT® 20 mcg/puff Metered Dose Inhaler ATROVENT® is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis, emphysema and asthma. For ATROVENT® 0.025% Nebuliser Solution ATROVENT® is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema. ATROVENT® is indicated, when used concomitantly with inhaled beta-agonists in the treatment of acute bronchospasm associated with chronic obstructive pulmonary disease including chronic bronchitis and asthma.
**Contraindications** ATROVENT® is contraindicated in patients with known hypersensitivity to atropine or its derivatives (such as the active substance ipratropium bromide) or to any other component of the product.
R03BB01
ipratropium bromide
Manufacturer Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
ISTITUTO DE ANGELI S.R.L. (IDAPH)
Active Ingredients
Documents
Package Inserts
Atrovent Solution PI.pdf
Approved: April 21, 2023
