Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** For international units (IU) and mass values, the following ratios apply: 1 mg benzylpenicillin sodium is equivalent to 1670 international units benzylpenicillin. 1 million international units benzylpenicillin is equivalent to 598.9 mg benzylpenicillin sodium. In general, 600 mg benzylpenicillin sodium is considered to be equivalent to 1 million international units benzylpenicillin. Benzylpenicillin has a wide dosage margin, which is guided by the method of administration, dose level and dosing interval according to pathogen type and susceptibility, severity of the infection and the patient’s condition. **Posology** In general, 30,000 international units (= 0.03 mega international units)/kg body weight daily, divided into 2 to 3 doses, are administered. _In general, the following dosage schedule should be followed;_ 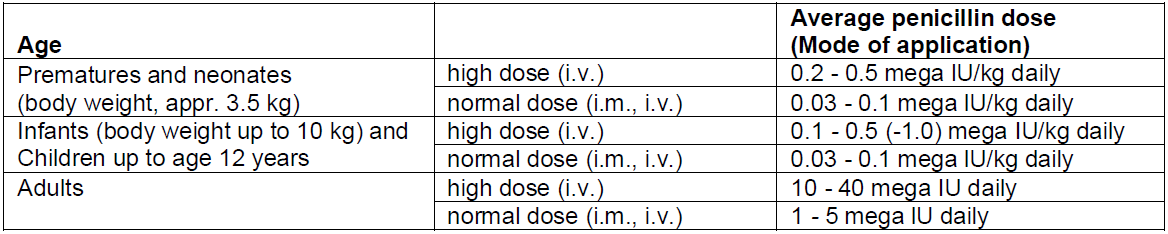 **Caution:** Cerebral seizures and electrolyte imbalance may occur if infusions are too rapid. A rate of not more than 300 mg/minute is recommended for intravenous doses above 1.2 g. In pre-term and newborn infants, the dosing interval must be no less than 12 hours due to immaturity and reduced excretion of benzylpenicillin (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Elderly: Elimination processes may be delayed with advanced age. The dosage must therefore be adjusted to renal function in each individual case (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Renal impairment If renal function is severely impaired, the degradation and excretion of penicillins may be delayed. This should be taken into account in the dosage. It is therefore recommended that the single doses and/or dosing intervals of Penicillin G Sodium be adjusted to the clearance values in each individual case: 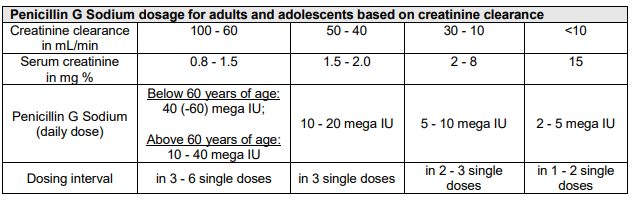 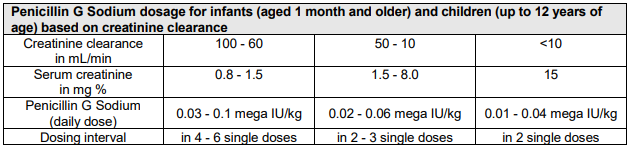 Infants (aged 1 month and older) and children (up to 12 years of age): If renal function is moderately-to-severely impaired (glomerular filtration rate = 10 – 50 mL/minute/1.73 m2), the normal dose is administered every 8 – 12 hours. In very severe cases of impaired renal function or renal failure (glomerular filtration rate <10 mL/minute/1.73 m2), the normal dose is administered every 12 hours. Pre-term and newborn infants (up to 4 weeks of age): Penicillin G Sodium is not suitable for the treatment of pre-term and newborn infants with impaired renal function. Hepatic impairment: No dose reduction is required provided that renal function is not impaired. Special dosages _Bacterial endocarditis:_Adults are given 10 – 80 mega international units/day intravenously in combination with aminoglycosides._Meningitis:_Due to increased seizure susceptibility and Jarisch-Herxheimer reactions, no more than 20 – 30 mega international units/day should be administered in adults and no more than 12 mega international units/day in children. Administration of the first dose should be protracted for very severe clinical forms - initially 1/4 of the individual single dose - to be given slowly under very strict surveillance._Lyme borreliosis:_In adults, 20 – 30 mega international units/day intravenously in 2 to 3 doses over 14 days and in children, 0.5 mega international units/kg/day intravenously in 2 – 3 doses over 14 days. _Special clinical situations:_ If required by the clinical situation, Penicillin G Sodium can also be administered as follows: - Intrapleural instillation: up to 0.2 mega international units (5,000 international units/mL) - Intra-articular injection: up to 0.1 mega international units (25,000 international units/mL) - Intrathecal administration: - Adults, adolescents (aged 12 years and older): 10,000 international units up to a maximum of 20,000 international units - Children (aged 6 – 12 years): 8,000 international units - Infants (aged 1 – 6 years): 5,000 international units - Infants (aged 1 – 23 months): 2,500 international units After withdrawing a corresponding amount of cerebrospinal fluid, the sterile solution (no more than 1,000 international units/ml) must be injected slowly (1 mL/min) at body temperature. Local therapy should always be merely adjuvant to systemic treatment. For intrathecal instillations, the dosage for systemic treatment (IV, IM) must be reduced accordingly. Method of administration Penicillin G Sodium can be given **intravenously** (injection or short infusion at 10 mega international units/100 mL) or also **intramuscularly**. _Notes for IM injection:_ Up to a maximum of 10 mega international units (= approximately 6 g) Penicillin G Sodium, dissolved in 6 – 10 mL water for injection, is applied up to twice daily as a deep intramuscular injection into the upper, outer quadrant of the gluteus maximus or Hochstetter's ventrogluteal field. 5 mL per injection site is to be regarded as the upper limit of tolerability. Repeated injections should be given on alternate sides. Higher doses can be given as an IV infusion. Severe local reactions may occur with intramuscular administration, especially in infants. If possible, intravenous therapy should be performed. **Caution**: Cerebral seizures and electrolyte imbalance may occur if the infusion is too rapid. A rate of not more than 300 mg/minute is recommended for intravenous doses above 1.2 g. For further information on preparation, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Duration of use The usual duration of treatment is 10 – 14 days for most indications. However, the duration of treatment should be adjusted to the severity of infection, sensitivity of the pathogen(s) and the patient’s clinical and bacteriological status. It should be continued for at least 2 – 3 days after the resolution of clinical symptoms. According to WHO recommendations, a treatment period of at least 10 days should be observed for streptococcal diseases.
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**4.1 Therapeutic indications** Penicillin G Sodium is indicated for the treatment of the following infections in adults, adolescents, children, newborn infants and pre-term infants, caused by penicillin-sensitive pathogens (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): - septicemia - skin and wound infections - diphtheria (in addition to antitoxin) - community acquired pneumonia - empyema - erysipelas - bacterial endocarditis - peritonitis - meningitis - brain abscesses - osteomyelitis - infections of the genital tract caused by fusobacteria. Penicillin G Sodium is also used for the treatment of the following specific infections: - anthrax - tetanus - listeriosis - pasteurellosis - rat bite fever - fusospirochetosis - actinomycosis Furthermore, Penicillin G Sodium is also used for complications in gonorrhea and syphilis (e.g. gonorrheal endocarditis or arthritis, congenital syphilis). However, in uncomplicated cases, preference should be given to depot penicillins. Penicillin G Sodium is not indicated for the treatment of syphilis during pregnancy. Penicillin G Sodium is also used in Lyme borreliosis from the second stage of the disease onwards (meningopolyneuritis Garin-Bujadoux-Bannwarth, acrodermatitis chronica atrophicans, Lyme arthritis, Lyme carditis) if oral penicillin therapy is no longer indicated. During pregnancy, high-dose parenteral Penicillin G Sodium administration is recommended from the second stage of Lyme disease onwards to prevent diaplacental infection. The generally acknowledged guidelines for the appropriate use of antibacterial agents should be considered when using Penicillin G Sodium.
**4.3 Contraindications** - Hypersensitivity to the active substance - History of hypersensitivity to penicillin - History of a severe immediate hypersensitivity reaction (e.g. anaphylaxis) to another beta-lactam agent (e.g. cephalosporin, carbapenem or monobactam)
J01CE01
benzylpenicillin
Manufacturer Information
SANDOZ SINGAPORE PTE. LTD.
SANDOZ GMBH
Active Ingredients
Documents
Package Inserts
Penicillin G Sodium Sandoz PI.pdf
Approved: October 31, 2022
