Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**Dosage and Administration** 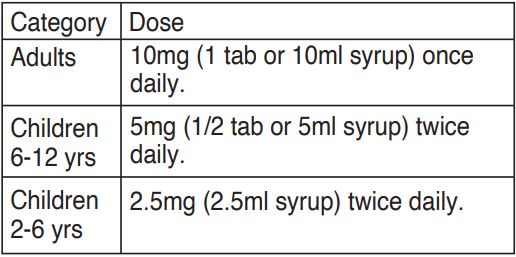 Cetirizine is safe for use in the elderly patients. Less frequent dosing is advised in patients with reduced creatinine clearance. In patients with renal insufficiency, dosage should be reduced to half the daily dose.
ORAL
Medical Information
**Indications** Atrizin® is indicated for the symptomatic relief of allergic manifestations, such as: - Seasonal allergic rhinitis - Perennial allergic rhinitis - Urticaria of allergic origin
**Contraindication** Cetirizine is contraindicated in patients who have shown hypersensitivity to it. It should not be administered to neonates, lactating mother and pregnant women.
R06AE07
cetirizine
Manufacturer Information
GOLDPLUS UNIVERSAL PTE LTD
BEXIMCO PHARMACEUTICALS LTD
