Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION, CONCENTRATE
**4.2 Dose and method of administration** **Dosage** Care in the administration of doxorubicin will reduce the chance of perivenous infiltration. It may also decrease the chance of local reactions such as urticaria and erythematous streaking. The recommended dosage schedule is 60–75 mg/m2 as a single intravenous injection administered at 21-day intervals. The lower dose should be given to patients with inadequate marrow reserves due to old age, or prior therapy, or neoplastic marrow infiltration. An alternative dose schedule is 30 mg/m2 on each of three successive days repeated every 4 weeks. The adult dosage regimens may be suitable for paediatric cases. The recommended lifetime cumulative dose limit is 550 mg doxorubicin/m2 body surface area. Doxorubicin has been administered as an intra-arterial infusion for 1–3 days at doses of 45–100 mg/m2. It is recommended that the total cumulative dose of doxorubicin for adults aged 70 or older be restricted to 450 mg/m2 body surface area. _**Use in hepatic impairment**_ Doxorubicin dosage must be reduced if hepatic function is impaired according to the following table: 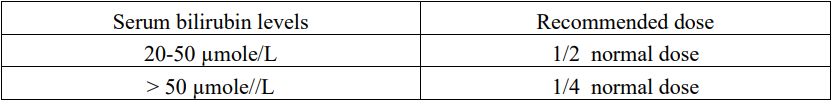 **Method of Administration** Doxorubicin is supplied as 10 mg and 50 mg doxorubicin hydrochloride in 5 and 25 mL vials, respectively (doxorubicin concentration 2 mg/mL). Doxorubicin must be handled with care. If contact with the skin occurs, wash thoroughly with soap and water. The product contains no antimicrobial preservative. The single dose vials should be used in one patient on one occasion only. Discard any residue. The solution is to be stored under refrigeration (2°C to 8°C) and should be protected from sunlight and retained in the carton until time of use. Storage of Doxorubicin at refrigerated conditions can result in the formation of a gelled product. This gelled product will return to a slightly viscous to mobile solution after two to a maximum of four hours equilibration at room temperature (15°C to 25°C). It is recommended that doxorubicin be slowly administered into the tubing of a freely running intravenous infusion of Sodium Chloride Injection U.S.P. or 5% Glucose Injection U.S.P. The tubing should be attached to a butterfly needle inserted preferably into a large vein. The rate of administration is dependent on the size of the vein and the dosage. However, the dose should be administered in not less than 3–5 minutes. A direct push injection is not recommended due to the risk of extravasation, which may occur even in the presence of adequate blood return upon needle aspiration. Local erythematous streaking along the vein as well as facial flushing may be indicative of too rapid administration. A burning or stinging sensation may be indicative of perivenous infiltration and the infusion should be immediately terminated and restarted in another vein. Doxorubicin has been used in combination with other approved chemotherapeutic agents. Though evidence is available that at least in some types of neoplastic disease combination chemotherapy is superior to single agents the benefits and risks of such therapy have not yet been fully elucidated. _**Protective measures**_ The following protective recommendations are given due to the toxic nature of this substance: - Personnel should be trained in good technique for reconstitution and handling. - Pregnant staff should be excluded from working with this drug. - Personnel handling doxorubicin should wear protective clothing: goggles, gowns and disposable gloves and masks. - A designated area should be defined for reconstitution (preferably under a laminar flow system). The work surface should be protected by disposable, plastic-backed, absorbent paper. - All items used for reconstitution, administration or cleaning, including gloves, should be placed in high-risk waste-disposal bags for high-temperature incineration. - Spillage or leakage should be treated with dilute sodium hypochlorite (1% available chlorine) solution, preferably by soaking, and then water. - All cleaning materials should be disposed of as indicated previously. - In case of skin contact thoroughly wash the affected area with soap and water or sodium bicarbonate solution. However, do not abrade the skin by using a scrub brush. - In case of contact with the eye(s), hold back the eyelid(s) and flush the affected eye(s) with copious amounts of water for at least 15 minutes. Then seek medical evaluation by a physician. - Always wash hands after removing gloves. _**Intravesical administration**_ The following procedure is recommended: 1. The bladder should be catheterised and emptied. 2. Dilute Doxorubicin to a final concentration of 80 mg in 100 mL of normal saline and instil via the catheter into the bladder. 3. The catheter should be removed, and the patient instructed to be on one side. At 15-minute intervals, the patient should alternate to the opposite side over a 1-hour period. 4. The patient should be requested not to urinate for 1 hour, after which the bladder should be emptied of solution. 5. The procedure should be repeated at monthly intervals.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** To produce regression in neoplastic conditions such as: acute leukaemia, Wilms’ tumour, neuroblastoma, soft tissue and bone sarcomas, breast carcinoma, lymphomas of both Hodgkin’s and non-Hodgkin’s type, bronchogenic (lung) carcinoma, thyroid carcinoma, hepatomas, ovarian carcinoma, etc. The main antitumour activities are listed in Table 1. Doxorubicin is also indicated by intravesical administration in the primary management of non-metastatic carcinoma of the bladder (Tis, T1, T2). 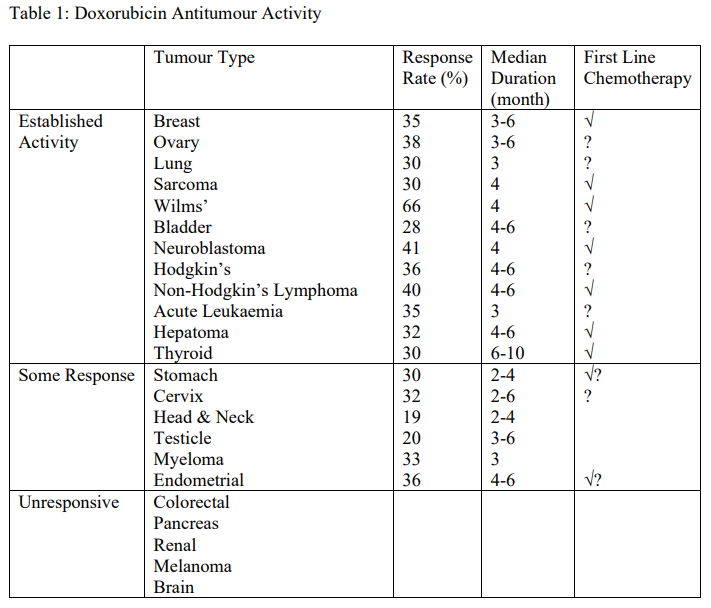
**4.3 Contraindications** Hypersensitivity to doxorubicin or any other component of the product, other anthracyclines or anthracenediones. Pregnancy and lactation (see Section 4.4 Special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Contraindications for intravenous administration:_ - persistent myelosuppression or severe stomatitis induced by previous treatment with other antitumour agents or by radiotherapy - general infection - severe impaired liver function - severe arrhythmia, impaired heart function, previous cardiac infarct - previous treatment with maximum cumulative doses of doxorubicin, daunorubicin, epirubicin, idarubicin and/or other anthracyclines and anthracenediones (see Section 4.4 Special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) _Contraindications for intravesical administration:_ - invasive tumours that have penetrated the bladder - urinary tract infections - inflammation of the bladder - problems with catheterisation
L01DB01
doxorubicin
Manufacturer Information
ADVAGEN PTE. LTD.
Sichuan Huiyu Pharmaceutical Co., Ltd
Active Ingredients
Documents
Package Inserts
DOXORUBICIN ADVAGEN_ PI_Feb 23.pdf
Approved: February 17, 2023
