Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**4.2 Posology and method of administration** Monitor carefully patients for signs and symptoms of hypersensitivity reactions during and following each administration of Venofer. Venofer should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be observed for adverse effects for at least 30 minutes following each Venofer injection. Posology The cumulative dose of Venofer must be calculated for each patient individually and must not be exceeded. _Calculation of dosage_ The total cumulative dose of Venofer, equivalent to the total iron deficit (mg), is determined by the haemoglobin level (Hb) and body weight (BW). The dose of Venofer must be individually calculated for each patient according to the total iron deficit calculated with the following Ganzoni formula, for example: 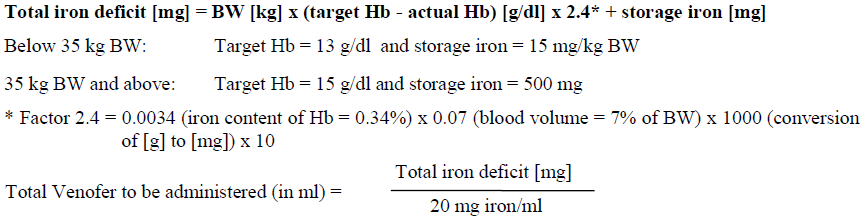 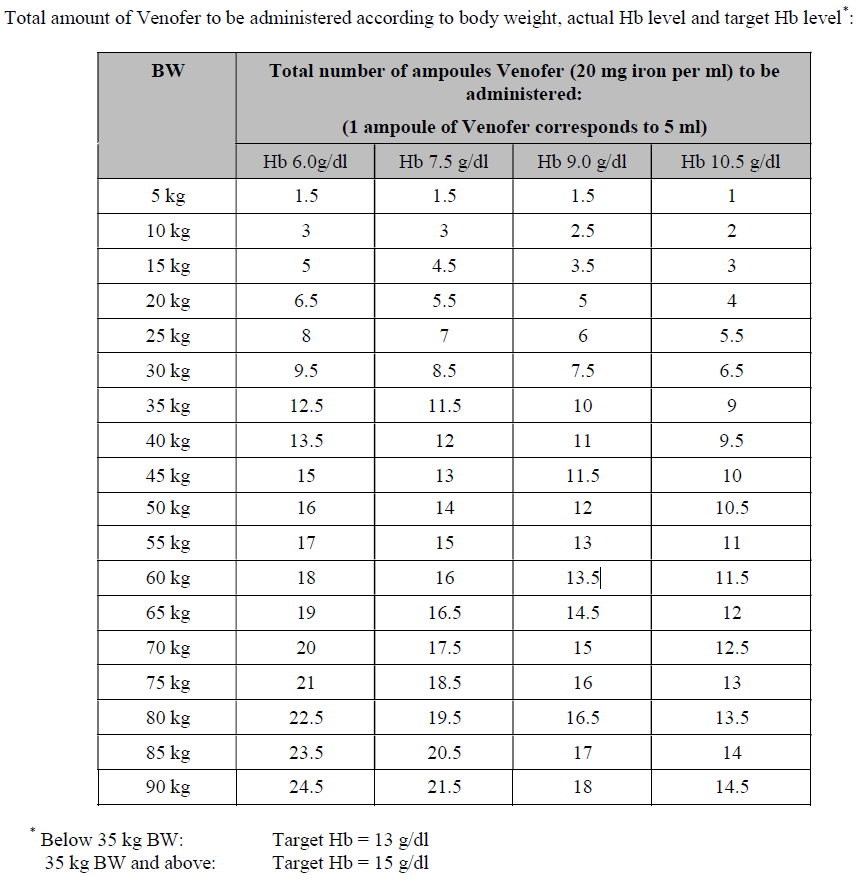 To convert Hb (mM) to Hb (g/dl), multiply the former by 1.6. If the total necessary dose exceeds the maximum allowed single dose, then the administration must be divided. If no response of the haematological parameters is observed after 1 to 2 weeks the original diagnosis should be reconsidered. _Calculation of dosage for iron replacement secondary to blood loss and to support autologous blood donation_ The required Venofer dose to compensate for the iron deficit may be calculated according the following formulas: If the quantity of blood lost is known: The administration of 200 mg iron (10 ml of Venofer) should result in an increase in Hb approximately equivalent to 1 unit blood (400 ml with Hb = 15 g/dl). 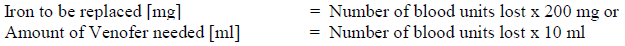 If the Hb level is less than desired: Formula assumes that the storage iron does not need to be restored. Iron to be replaced \[mg\] = BW \[kg\] x 2.4 x (target Hb – actual Hb) \[g/dl\] 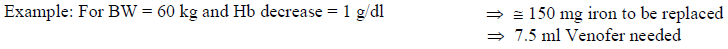 For the maximum tolerated single and weekly dose, see “Normal posology” and “Maximum tolerated single and weekly doses”. _Normal posology:_ _Adults_ 5 – 10 ml of Venofer (100 – 200 mg iron) 1 to 3 times a week. For administration time and dilution ratio see “Method of administration”. _Paediatric population_ There is moderate amount of data in children under study conditions. If there is a clinical need, it is recommended not to exceed 0.15 ml of Venofer (3 mg iron) per kg body weight not more than three times per week. For administration time and dilution ratio see “Method of administration”. _Maximum tolerated single and weekly doses_ _Adults_ As an injection, maximum tolerated dose per day given not more than 3 times per week: - 10 ml of Venofer (200 mg iron) injected over at least 10 minutes As an infusion, maximum tolerated dose per day given not more than once per week: - Patients above 70 kg body weight: 500 mg iron (25 ml of Venofer) over at least 3 ½ hours - Patients of 70 kg body weight and below: 7 mg iron/kg body weight over at least 3 ½ hours The infusion times given in “Method of administration” should be strictly adhered to, even if the patient does not receive the maximum tolerated single dose. Method of administration Venofer must only be administered by the intravenous route. This may be by drip infusion, slow injection or directly into the venous line of the dialysis machine. _Intravenous drip infusion_ Venofer must only be diluted in sterile 0.9% m/V sodium chloride (NaCl) solution. Dilution must take place immediately prior to infusion and the solution should be administered as follows: 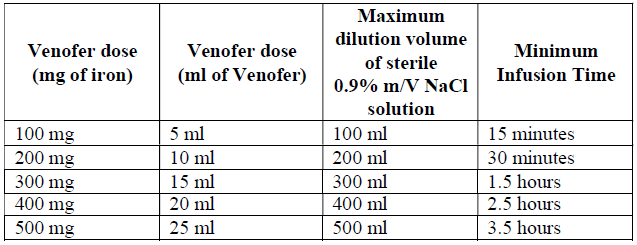 _Intravenous injection_ Venofer may be administered by slow intravenous injection at a rate of 1 ml undiluted solution per minute and not exceeding 10 ml (200 mg iron) per injection. _Injection into venous line of dialysis machine_ Venofer may be administered during a haemodialysis session directly into the venous line of the dialysis machine under the same conditions as for intravenous injection.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Venofer is indicated for the treatment of iron deficiency in the following indications: - Where there is a clinical need for a rapid iron supply - In patients who cannot tolerate oral iron therapy or who are non-compliant - Where oral iron preparations are ineffective (e.g., in active inflammatory bowel disease) Venofer should only be administered where the indication is confirmed by appropriate investigations.
**4.3 Contraindications** The use of Venofer is contraindicated in the following conditions: - Hypersensitivity to iron sucrose, Venofer or to any of its excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ - Anaemia not caused by iron deficiency - Evidence of iron overload or disturbances in utilisation of iron - Pregnancy first Trimester
B03AC
非肠道用药的三价铁制剂
Manufacturer Information
VIFOR PHARMA ASIA PACIFIC PTE. LTD.
IDT Biologika GmbH
Takeda Austria GmbH
Active Ingredients
Documents
Package Inserts
Venofer PI.pdf
Approved: November 3, 2021
