Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
_**3\. How to use Insulatard®**_ Talk about your insulin needs with your doctor and nurse. Make sure you take Insulatard® Penfill® as instructed by your doctor or nurse and follow their advice carefully. If your doctor has switched you from one type or brand of insulin to another, your dose may have to be adjusted by your doctor. It is recommended that you measure your blood sugar regularly. **How to use this insulin** **Insulatard® is administered by injection under the skin** (subcutaneously). Never inject your insulin directly into a vein or muscle. Always vary the sites you inject within the same region, to reduce the risk of developing lumps or skin pitting (see _5 Possible side effects_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The best places to give yourself an injection are: your buttocks; the front of your thighs or upper arms. **Resuspending the insulin** Resuspending is easier when the insulin has reached room temperature. Before you put the Penfill® cartridge into the insulin delivery system, move it up and down between positions **a** and **b** and back (see the picture) so that the glass ball moves from one end of the cartridge to the other at least 20 times. Repeat this movement at least 10 times before each injection. The movement must always be repeated until the liquid appears uniformly white and cloudy. Complete the other stages of injection without delay. 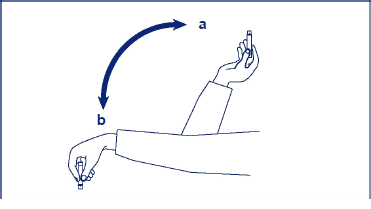 **Check there are at least 12 units** of insulin left in the cartridge to allow even resuspending. If there are less than 12 units left, use a new one. **How to inject this insulin** - **Inject the insulin** under the skin. Use the injection technique advised by your doctor or nurse and as described in your delivery system manual. - **Keep the needle under your skin** for at least 6 seconds. Keep the push-button fully depressed until the needle has been withdrawn. This will ensure correct delivery and limit possible flow of blood into the needle or insulin reservoir. - **After each injection** be sure to remove and discard the needle and store Insulatard® without the needle attached. Otherwise the liquid may leak out which can cause inaccurate dosing. Do not refill Insulatard® Penfill®. Penfill® cartridges are designed to be used with Novo Nordisk insulin delivery systems and NovoFine® or NovoTwist® needles. If you are treated with Insulatard® Penfill® and another insulin Penfill® cartridge, you should use two insulin delivery systems, one for each type of insulin. As a precautionary measure, always carry a spare insulin delivery system in case your Penfill® is lost or damaged.
SUBCUTANEOUS
Medical Information
_**1\. What Insulatard® is and what it is used for**_ **Insulatard® is human insulin used to treat diabetes.** Diabetes mellitus is a disease where your body does not produce enough insulin to control the level of your blood sugar. Insulatard® is a long-acting insulin. This means that it will start to lower your blood sugar about 1½ hours after you take it, and the effect will last for approximately 24 hours. Insulatard® is often given in combination with fast-acting insulin products.
_**2\. Before you use Insulatard®**_ **Do not use Insulatard®** - **In insulin infusion pumps.** - **If you are allergic (hypersensitive)** to human insulin or any of the other ingredients in Insulatard® (see _7 Further information_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **If you suspect hypoglycaemia** (low blood sugar) is starting (see _4 What to do in an emergency_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **If the cartridge or the device containing the cartridge is dropped, damaged or crushed.** - **If it has not been stored correctly** or been frozen (see _6 How to store Insulatard®_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **If the resuspended insulin does not appear uniformly white and cloudy.**
A10AB01
insulin (human)
Manufacturer Information
NOVO NORDISK PHARMA (SINGAPORE) PTE LTD
Novo Nordisk A/S
Novo Nordisk Producao Farmaceutica do Brasil Ltda
Novo Nordisk Production SAS
Active Ingredients
Documents
Patient Information Leaflets
Insulatard Penfill Injection PIL.pdf
Approved: November 22, 2021
