Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**DOSAGE REGIMEN AND ADMINISTRATION** Afinitor Tablets may be used for the treatment of patients with TSC who have SEGA in conjunction with therapeutic drug monitoring (see sub-section Therapeutic drug monitoring and section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Treatment with Afinitor should be initiated by a physician experienced in the use of anticancer therapies or in the treatment of patients with TSC. Treatment should continue as long as clinical benefit is observed or until unacceptable toxicity occurs. **General target population** **Adults** **Dosing in hormone receptor-positive advanced breast cancer, advanced neuroendocrine tumours of gastrointestinal, lung or pancreatic origin, advanced renal cell carcinoma and TSC with renal angiomyolipoma** The recommended dose of Afinitor is 10 mg, to be taken once daily (see section METHOD OF ADMINISTRATION). **Dosing in TSC with SEGA:** Individualize dosing based on body surface area (BSA, in m2) using the Dubois formula, where weight (W) is in kilograms and height (H) is in centimeters: 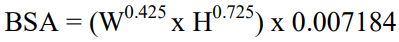 Starting dose and target trough concentrations in TSC with SEGA The recommended starting daily dose for Afinitor for the treatment of patients with TSC who have SEGA is 4.5 mg/m2, rounded to the nearest strength of Afinitor Tablets. Different strengths of Afinitor Tablets can be combined to attain the desired dose. Dosing should be titrated to attain trough concentrations of 3 to 15 ng/mL. **Dose monitoring** Therapeutic drug monitoring of everolimus blood concentrations is required for patients with TSC who have SEGA (see section Therapeutic Drug Monitoring). Everolimus whole blood trough concentrations should be assessed approximately 1 to 2 weeks after commencing treatment or any change in dose. **Titration** Individualized dosing should be titrated by increasing the dose by increments of 1 to 4 mg to attain the target trough concentration for optimal clinical response. Efficacy, safety, concomitant medication, and the current trough concentration should be considered when planning for dose titration. Individualized dose titration can be based on simple proportion: 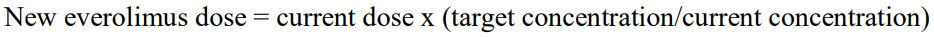 For example, a patient’s current dose based on BSA is 4 mg with a steady state concentration of 4 ng/mL. In order to achieve a target concentration above the lower Cmin limit of 5 ng/mL, e.g. 8 ng/mL, the new everolimus dose would be 8 mg (an increase of 4 mg to the current daily dose). The trough concentration should then be assessed 1 to 2 weeks after this change in dose. **Long-term dose monitoring** For patients with TSC who have SEGA, evaluate SEGA volume approximately 3 months after commencing Afinitor therapy, with subsequent dose adjustments taking into consideration changes in SEGA volume, corresponding trough concentration, and tolerability (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with TSC who have SEGA, once a stable desired dose is attained, monitor trough concentrations every 3 to 6 months in patients with changing body surface area or every 6 to 12 months in patients with stable body surface area for the duration of treatment. **Dose Modifications** **Adverse drug reactions:** Management of severe or intolerable adverse drug reactions (ADR) may require temporary dose interruption (with or without dose reduction) or discontinuation of Afinitor therapy. If dose reduction is required, the suggested dose is approximately 50% lower than the daily dose previously administered (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For dose reductions below the lowest available tablet strength, alternate day dosing should be considered. Table 1 summarizes recommendations for dose interruption, reduction or discontinuation of Afinitor in the management of ADRs. General management recommendations are also provided as applicable. Clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment. 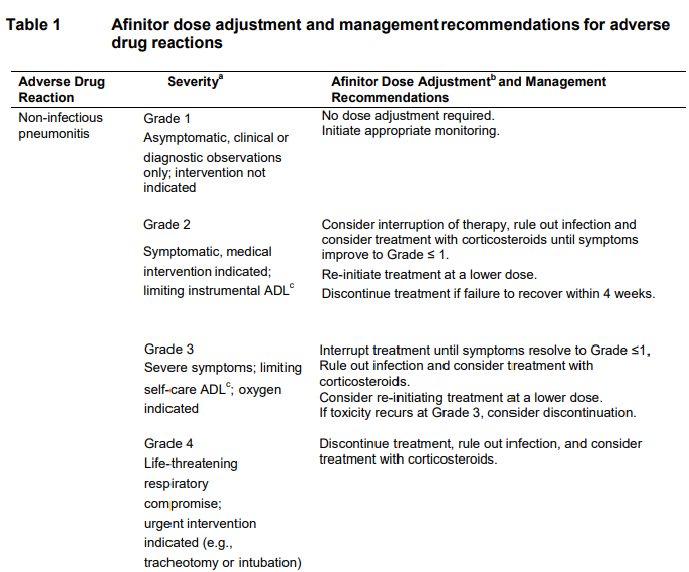 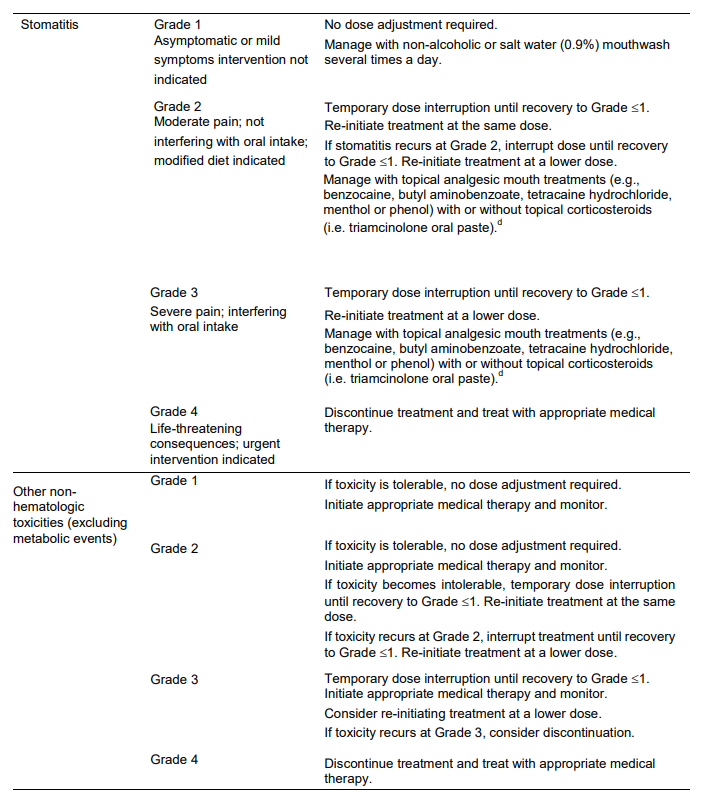 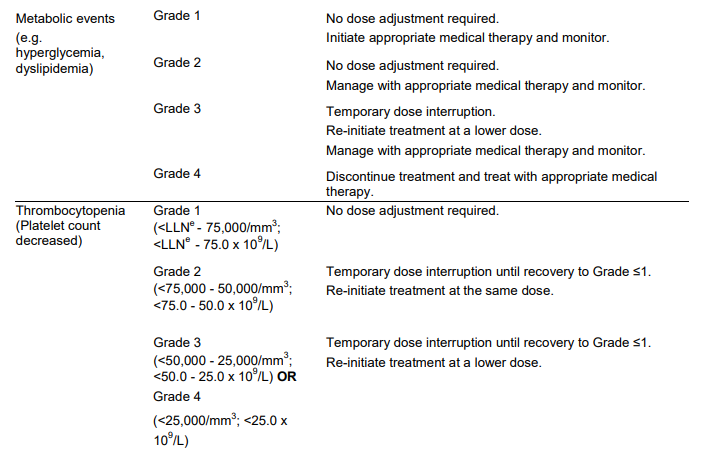 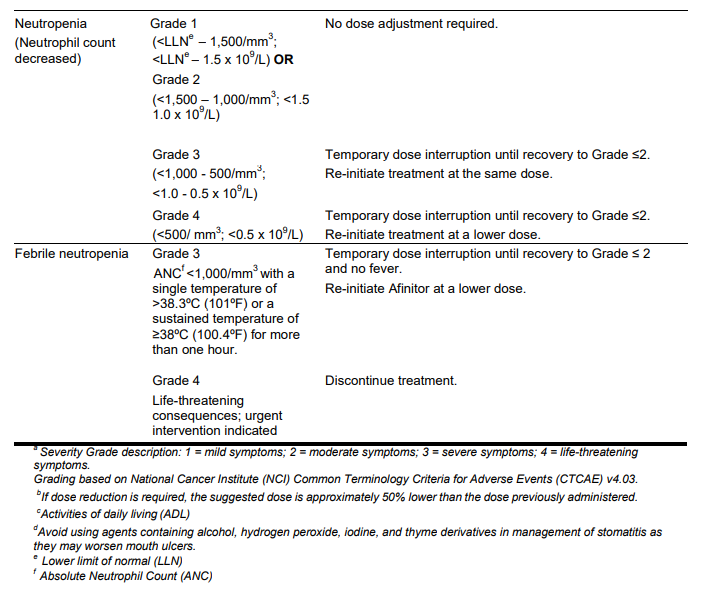 **Moderate CYP3A4/PgP inhibitors** Use caution when administering Afinitor in combination with moderate CYP3A4/PgP inhibitors. If patients require co-administration of a moderate CYP3A4/PgP inhibitor, reduce the dose by approximately 50%. Further dose reduction may be required to manage ADRs. For dose reductions below the lowest available strength, alternate day dosing should be considered (see section WARNINGS AND PRECAUTIONS and section INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **Hormone receptor-positive advanced breast cancer, advanced neuroendocrine tumours of gastrointestinal, lung or pancreatic origin, advanced renal cell carcinoma, and TSC with renal angiomyolipoma:** If the moderate CYP3A4/PgP inhibitor is discontinued, consider a washout period of at least 2 to 3 days (average for most commonly used moderate inhibitors) before the Afinitor dose is increased. The Afinitor dose should be returned to the dose used prior to initiation of the moderate CYP3A4/PgP inhibitor (see section WARNING AND PRECAUTIONS and section INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **TSC with SEGA:** Everolimus trough concentrations should be assessed approximately 1 to 2 weeks after the addition of a moderate CYP3A4/PgP inhibitor. If the inhibitor is discontinued the Afinitor dose should be returned to the dose used prior to initiation of the inhibitor and the everolimus trough concentration should be re-assessed approximately 2 weeks later (see sub-section Therapeutic drug monitoring, WARNINGS AND PRECAUTIONS and INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Strong CYP3A4 inducers** Avoid the use of concomitant strong CYP3A4 inducers. - **Hormone receptor-positive advanced breast cancer, advanced neuroendocrine tumours of gastrointestinal, lung or pancreatic origin, advanced renal cell carcinoma, and TSC with renal angiomyolipoma:** If patients require co-administration of a strong CYP3A4 inducer, consider doubling the daily dose of Afinitor (based on pharmacokinetic data), using increments of 5 mg or less. This dose of Afinitor is predicted to adjust the AUC to the range observed without inducers. However, there are no clinical data with this dose adjustment in patients receiving strong CYP3A4 inducers. If the strong inducer is discontinued, consider a washout period of at least 3 to 5 days (reasonable time for significant enzyme de-induction), before the Afinitor dose is returned to the dose used prior to initiation of the strong CYP3A4 inducer (see section WARNINGS AND PRECAUTIONS and section INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **TSC with SEGA:** Patients with SEGA receiving concomitant strong CYP3A4 inducers (e.g., the enzyme inducing antiepileptic drugs carbamazepine, phenobarbital, and phenytoin) at the start of treatment may require an increased Afinitor dose to attain trough concentrations of 3 to 15 ng/mL. Double the daily dose of Afinitor and assess tolerability. Assess the everolimus trough level approximately two weeks after doubling the dose. Further adjust the dose by increments of 1 to 4 mg as necessary to maintain the target trough concentrations. - For SEGA patients not receiving concomitant strong inducers at the start of everolimus treatment, the addition of a strong inducer may require an increased Afinitor dose. Double the daily dose of Afinitor and assess tolerability. Assess the everolimus trough level two weeks after doubling the dose. Further adjust the dose if necessary by increments of 1 to 4 mg as necessary to maintain the target trough concentration. - The addition of another concomitant strong CYP3A4 inducer may not require additional dose adjustment. Assess the everolimus trough level two weeks after initiating the additional inducer. Adjust the dose in 1 to 4 mg increments as necessary to maintain the target trough concentration. - Discontinuation of one of multiple strong CYP3A4 inducers may not require additional dose adjustment. Assess the everolimus trough level two weeks after discontinuation of one of multiple strong CYP3A4 inducers. If all strong inducers are discontinued consider a washout period of at least 3 to 5 days (reasonable time for significant enzyme de-induction) before the Afinitor dose is returned to the dose used prior to initiation of the strong CYP3A4 inducer. Assess the everolimus trough concentrations approximately two weeks later (see sections Therapeutic drug monitoring, WARNINGS AND PRECAUTIONS and INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Special populations** **Paediatric patients (below 18 years)** - Afinitor is not recommended for use in paediatric cancer patients. - Afinitor is not recommended for use in paediatric patients with TSC who have renal angiomyolipoma. Afinitor has not been studied in pediatric patients <1 year of age with TSC who have SEGA. - Dosing recommendations for pediatric patients with TSC who have SEGA are consistent with those for the corresponding adult population with the exception of those patients with hepatic impairment. Afinitor is not recommended for patients <18 years of age with hepatic impairment and TSC with SEGA. **Geriatrics patients (65 years of age or older)** No dosage adjustment is required (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal impairment** No dosage adjustment is required (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** **Hormone receptor-positive advanced breast cancer, advanced neuroendocrine tumours of gastrointestinal, lung or pancreatic origin, advanced renal cell carcinoma and TSC with renal angiomyolipoma:** - Mild hepatic impairment (Child-Pugh A) – the recommended dose is 7.5 mg daily. - Moderate hepatic impairment (Child-Pugh B) – the recommended dose is 5 mg daily; the dose may be decreased to 2.5 mg if not well tolerated. - Severe hepatic impairment (Child-Pugh C) – not recommended. If the desired benefit outweighs the risk, a dose of 2.5 mg daily must not be exceeded. Dose adjustments should be made if a patient’s hepatic (Child-Pugh) status changes during treatment. **TSC with SEGA:** **Patients ≥18 years of age** - Mild hepatic impairment (Child-Pugh A) – 75% of the dose calculated based on BSA (rounded to the nearest strength) - Moderate hepatic impairment (Child-Pugh B) – 50% of the dose calculated based on BSA (rounded to the nearest strength) - Severe hepatic impairment (Child-Pugh C) – not recommended. If the desired benefit outweighs the risk, 25% of the dose calculated based on BSA (rounded to the nearest strength) must not be exceeded. Everolimus whole blood trough concentrations should be assessed approximately 1 to 2 weeks after commencing treatment or after any change in hepatic (Child-Pugh) status. For patients with SEGA, dosing should be titrated to attain trough concentrations of 3 to 15 ng/mL (see section Therapeutic drug monitoring). Dose adjustments should be made if a patient’s hepatic (Child-Pugh) status changes during treatment (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Patients <18 years of age** - Afinitor is not recommended for patients <18 years of age with TSC with SEGA and hepatic impairment. **Therapeutic drug monitoring** Therapeutic drug monitoring of everolimus blood concentrations is required for patients treated for TSC with SEGA using a validated bioanalytical LC/MS method. When possible, use the same assay and laboratory for therapeutic drug monitoring throughout treatment. Trough concentrations should be assessed approximately 1 to 2 weeks after the initial dose, after any change in dosage form, after an initiation or change in co-administration of CYP3A4/PgP inducers and/or inhibitors (see sections WARNINGS AND PRECAUTIONS and INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), or after any change in hepatic (Child-Pugh) status (see sections DOSAGE REGIMEN AND ADMINISTRATION and CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Trough concentrations should be assessed approximately 2 weeks after initiation or change in co-administration of CYP3A4/PgP inducers (see sections WARNINGS AND PRECAUTIONS and INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dosing should be titrated with the objective of attaining everolimus trough concentrations of 3 to 15 ng/mL, for patients with TSC who have SEGA, subject to tolerability (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The dose may be increased to attain a higher trough concentration within the target range to obtain optimal efficacy, subject to tolerability. **Method of Administration** Afinitor should be administered orally once daily at the same time every day, either consistently with or consistently without food (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Afinitor Tablets should be swallowed whole with a glass of water. The tablets should not be chewed or crushed. For patients with TSC who have SEGA and are unable to swallow tablets whole, Afinitor Tablet(s) can be dispersed completely in a glass of water (containing approximately 30 mL) by gently stirring until the tablet(s) is fully disintegrated (approximately 7 minutes), immediately prior to drinking. The glass should be rinsed with the same volume of water and the rinse completely swallowed to ensure the entire dose is administered (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Missed dose** Afinitor can still be taken up to 6 hours after the time it is normally taken. After more than 6 hours, the dose should be skipped for that day. The next day, Afinitor should be taken at its usual time. Double doses should not be taken to make up for the one that was missed.
ORAL
Medical Information
**INDICATIONS** Afinitor is indicated for the treatment of: - Hormone receptor-positive, HER2/neu negative advanced breast cancer, in combination with exemestane, in postmenopausal women without symptomatic visceral disease after recurrence or progression following a non-steroidal aromatase inhibitor. - Progressive neuroendocrine tumours of pancreatic origin (PNET) in patients with unresectable, locally advanced or metastatic disease. - Unresectable or metastatic, well-differentiated (Grade 1 or 2) non-functional neuroendocrine tumours of gastrointestinal or lung origin in adults with progressive disease. - Patients with advanced renal cell carcinoma whose disease has progressed on or after treatment with VEGF-targeted therapy. - Adult patients (≥18 years of age) with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. - Patients with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention, but are not candidates for curative surgical resection. The effectiveness of AFINITOR is based on an analysis of change in SEGA volume. Clinical benefit, such as improvement in disease-related symptoms or increase in overall survival, has not been demonstrated.
**CONTRAINDICATIONS** Afinitor is contraindicated in patients with hypersensitivity to the active substance, to other rapamycin derivatives or to any of the excipients (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01XE10
xl 01 xe 10
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Novartis Pharma Stein AG
Lek Pharmaceuticals d.d. (drug product intermediate manufacturer)
Novartis Pharma AG (drug product intermediate manufacturer)
Active Ingredients
Documents
Package Inserts
Afinitor PI.pdf
Approved: November 30, 2021
