Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SUSPENSION
**POSOLOGY AND METHOD OF ADMINISTRATION** **Posology:** Adults: 1 rectal suspension at bedtime. Paediatric population: There is little experience and only limited documentation for an effect in children. **Method of administration:** 1. A visit to the toilet is recommended before administration of the rectal suspension. 2. Immediately before use take the rectal suspension bottle out of the aluminium foil pack and shake it well (Figure 1). 3. To break the seal, twist the nozzle clockwise one full turn (the nozzle should then be in the same direction as before turning) (Figure 2). 4. Put your hand in one of the plastic disposal bags provided in the pack (Figure 3). 5. Hold the container as shown in the picture (Figure 4). Lubricate top part of rectal applicator. 6. To administer the rectal suspension, lie on your left side with the left leg straight and the right leg bent forward for balance. Carefully insert the applicator tip into the rectum. Maintain sufficient steady hand pressure while dispersing the bottle content. The bottle content should be applied within max. 30–40 seconds (Figure 5). 7. Once the bottle is empty, withdraw the tip with the bottle still compressed. 8. The rectal suspension should be retained in the bowel. Remain relaxed in the administration position for 5–10 minutes or until the urge to pass the rectal suspension has disappeared (Figure 6). Try to retain the rectal suspension overnight. 9. Roll the plastic disposal bag over the empty bottle (Figure 7). Discard it and wash your hands. 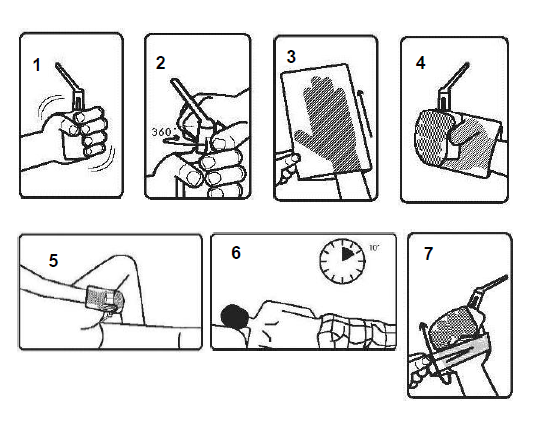
RECTAL
Medical Information
**THERAPEUTIC INDICATIONS** Treatment of ulcerative proctosigmoiditis.
**CONTRAINDICATIONS** Hypersensitivity to mesalazine, any of the excipients, or salicylates. Severe liver or renal impairment.
A07EC02
mesalazine
Manufacturer Information
FERRING PHARMACEUTICALS PRIVATE LIMITED
Ferring-Léčiva, a.s.
Active Ingredients
Documents
Package Inserts
Pentasa Rectal Suspension PI_PENSUS-I-SG-04.01_Proposed Clean.pdf
Approved: June 7, 2021
