Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**Dosage and Administration** Pharmaceutical form: Powder for concentrate for solution for infusion Treatment with _BLENREP_ should be initiated and supervised by physicians experienced in the treatment of multiple myeloma. **Method of Administration** _BLENREP_ is a cytotoxic anticancer medicinal product. Proper handling procedures should be followed. Instructions on reconstitution and further dilution are provided in _Use and Handling_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. _BLENREP_ is administered as an intravenous infusion over approximately 30 minutes. **Recommended Supportive Care** Patients should have an ophthalmic examination (including visual acuity and slit lamp examination) performed by an eye care professional at baseline, before the subsequent 3 treatment cycles, and as clinically indicated whilst on treatment ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Physicians should advise patients to administer preservative-free artificial tears at least 4 times a day beginning on the first day of infusion and continuing until completion of treatment as this may reduce corneal symptoms ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with dry eye symptoms, additional therapies may be considered as recommended by their eye care professional. **Adults** The recommended dosage is 2.5 mg/kg belantamab mafodotin administered as an intravenous infusion once every 3 weeks. _Dose Modifications_ Recommended dose modifications are provided in Table 1 for corneal adverse reactions and in Table 2 for other adverse reactions. Corneal adverse reactions may include findings upon eye examination and/or changes in visual acuity ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The treating physician should review the patient’s ophthalmic examination report before dosing and determine the dose of _BLENREP_ based on the highest category from the report in the most severely affected eye as both eyes may not be affected to the same degree (Table 1). During the ophthalmic examination, the eye care professional should assess the following: - The corneal examination finding(s) and the decline in best corrected visual acuity (BCVA). - If there is a decline in BCVA, the relationship of corneal examination findings to _BLENREP_ should be determined. - The highest category grading for these examination findings and BCVA should be reported to the treating physician.  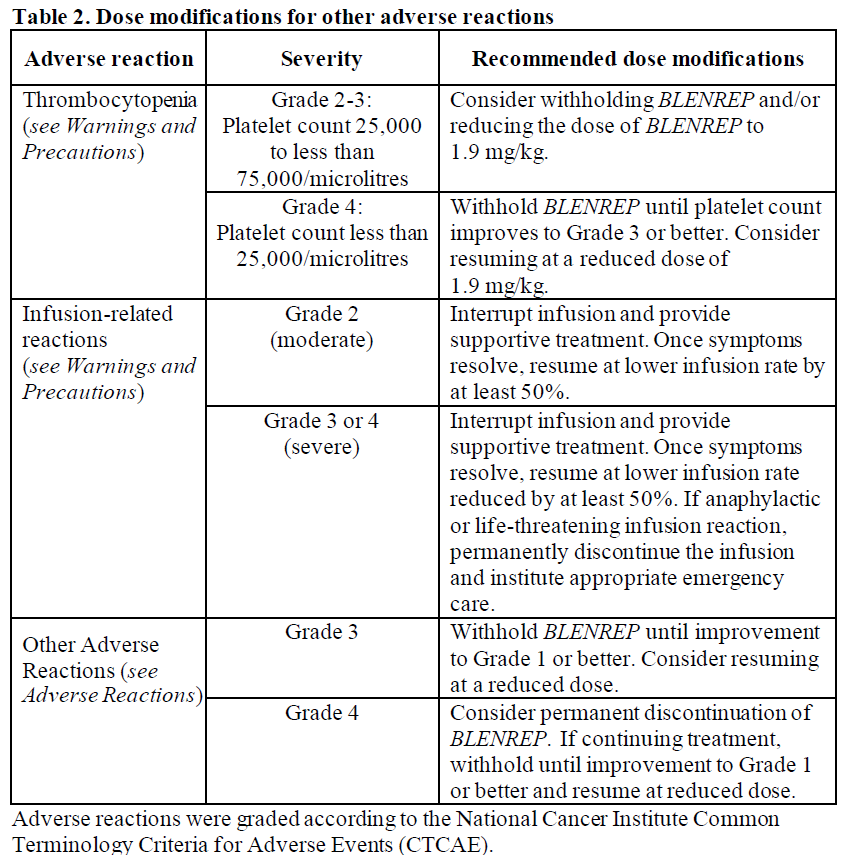 **Children** The safety and efficacy of _BLENREP_ have not been established in children less than 18 years of age. **Elderly** No dosage adjustment is required in patients over 65 years of age ( _see Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal impairment** No dose adjustment is required in patients with mild or moderate renal impairment (eGFR ≥30 mL/min/1.73m2). There are insufficient data in patients with severe renal impairment to support a dose recommendation ( _See Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** No dose adjustment is required in patients with mild hepatic impairment (bilirubin greater than ULN to less than or equal to 1.5 × ULN or aspartate transaminase \[AST\] greater than ULN). There are insufficient data in patients with moderate hepatic impairment and no data in patients with severe hepatic impairment to support a dose recommendation ( _see Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**Indications** _BLENREP_ is indicated as monotherapy for the treatment of multiple myeloma in adult patients, who have received at least 4 prior therapies and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.
**Contraindications** Hypersentivity to the active substance or to any of the excipients.
L01XC39
xl 01 xc 39
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
Baxter Oncology GmbH
Active Ingredients
Documents
Package Inserts
Blenrep 100 mg PI.pdf
Approved: September 21, 2022
