Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**DOSAGE AND ADMINISTRATION** **ADULTS** **Rheumatoid Arthritis, Psoriatic Arthritis and Ankylosing Spondylitis** The recommended dose of adalimumab for adult patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis is 40 mg administered every other week as a single dose via subcutaneous injection. Methotrexate, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs, analgesics or other DMARDs may be continued during treatment with adalimumab. In rheumatoid arthritis, some patients not taking concomitant MTX may derive additional benefit from increasing the dosage of adalimumab to 40 mg every week or 80 mg every other week. **Crohn’s Disease** 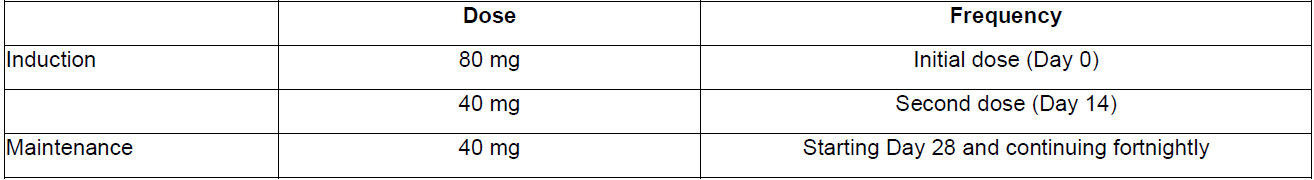 In case there is a need for a more rapid response to therapy, the regimen 160 mg at week 0 (given as 160 mg in one day or as 80 mg per day for two consecutive days), 80 mg at week 2, can be used with the awareness that the risk for adverse events is higher during induction. Some patients who experience a decrease in their response may benefit from an increase in dosage to 40 mg adalimumab every week or 80 mg every other week. Aminosalicylates, corticosteroids, and/or immunomodulatory agents (e.g., 6-mercaptopurine and azathioprine) may be continued during treatment with adalimumab. Patients usually respond within the induction phase. However, if a patient does not show any response, available data do not sufficiently support further adalimumab treatment. **Ulcerative Colitis** The recommended adalimumab induction dose regimen for adult patients with moderate to severe ulcerative colitis is 160 mg at Week 0 (given as 160 mg in one day or as 80 mg per day for two consecutive days) and 80 mg at Week 2. After induction treatment, the recommended dose is 40 mg every other week via subcutaneous injection. Aminosalicylates, corticosteroids, and/or immunomodulatory agents (e.g., 6-mercaptopurine and azathioprine) may be continued during treatment with adalimumab. During maintenance treatment, corticosteroids may be tapered in accordance with clinical practice guidelines. Some patients who experience decrease in their response may benefit from an increase in dosage to 40 mg adalimumab every week or 80 mg every other week. Available data suggest that clinical response is usually achieved within 2–8 weeks of treatment. Adalimumab should only be continued in patients who have responded during the first 8 weeks of therapy. **Plaque Psoriasis** The recommended dose of adalimumab for adult patients with plaque psoriasis is an initial dose of 80 mg, followed by 40 mg given every other week starting one week after the initial dose. Continued therapy beyond 16 weeks should be carefully reconsidered in a patient not responding within this time period. Beyond 16 weeks, patients with inadequate response may benefit from an increase in dosage to 40 mg every week or 80 mg every other week. Response should be periodically evaluated (for example, every 12 weeks). Patients with continued inadequate response should discontinue treatment. If an adequate response is achieved with an increased dosage, the dose may subsequently be reduced to 40 mg fortnightly. **Hidradenitis Suppurativa** The recommended adalimumab dose regimen for adult patients with hidradenitis suppurativa (HS) is 160 mg initially at Day 1 (given as 160 mg in one day or as 80 mg per day for two consecutive days), followed by 80 mg two weeks later at Day 15. Two weeks later (Day 29) continue with a dose of 40 mg every week or 80 mg every other week. Antibiotics may be continued during treatment with adalimumab if necessary. It is recommended that the patient should use a topical antiseptic wash on their HS lesions on a daily basis during treatment with adalimumab. Should treatment need to be interrupted, adalimumab 40 mg every week may be re-introduced. In patients without any benefit after 12 weeks of treatment, continued therapy should be reconsidered. **Uveitis** Ophthalmologists are advised to consult with an appropriate specialist before initiation of treatment with adalimumab. The recommended dose of adalimumab for adult patients with uveitis is an initial dose of 80 mg, followed by 40 mg given every other week starting one week after the initial dose. There is limited experience in the initiation of treatment with adalimumab alone. Treatment with adalimumab can be initiated in combination with corticosteroids and/or with other non-biologic immunomodulatory agents. Concomitant corticosteroids may be tapered in accordance with clinical practice starting two weeks after initiating treatment with adalimumab. It is recommended that the benefit and risk of continued long-term treatment should be evaluated on a yearly basis. **PEDIATRICS** **Juvenile Idiopathic Arthritis** **Polyarticular Juvenile Idiopathic Arthritis** The recommended dose of adalimumab for patients from 2 years of age with polyarticular juvenile idiopathic arthritis (JIA) is based on body weight (see Table 36). MTX, glucocorticoids, NSAIDs, and/or analgesics may be continued during treatment with adalimumab. Adalimumab may be available in different strengths and/or presentations. 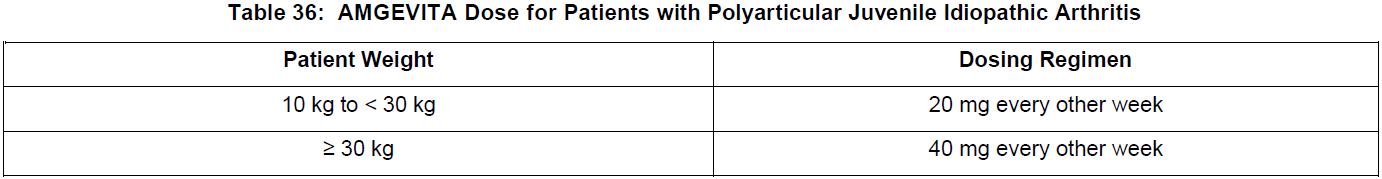 Adalimumab has not been studied in patients with polyarticular juvenile idiopathic arthritis less than 2 years of age or in patients with a weight below 10 kg. Available data suggest that clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be carefully reconsidered in a patient not responding within this time period. There is no relevant use of adalimumab in children aged less than 2 years in this indication. **Enthesitis-Related Arthritis** The recommended dose of adalimumab for patients from 6 years of age with enthesitis-related arthritis is based on body weight (see Table 37). Adalimumab may be available in different strengths and/or presentations. 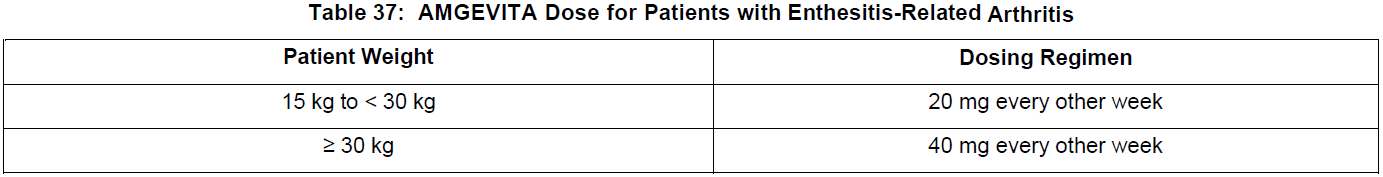 Adalimumab has not been studied in patients with enthesitis-related arthritis aged less than 6 years. **Pediatric Crohn’s Disease** The recommended dose of adalimumab for patients from 6 to 17 years of age with Crohn’s disease is based on body weight (see Table 38). Adalimumab is administered via subcutaneous injection. Adalimumab may be available in different strengths and/or presentations. 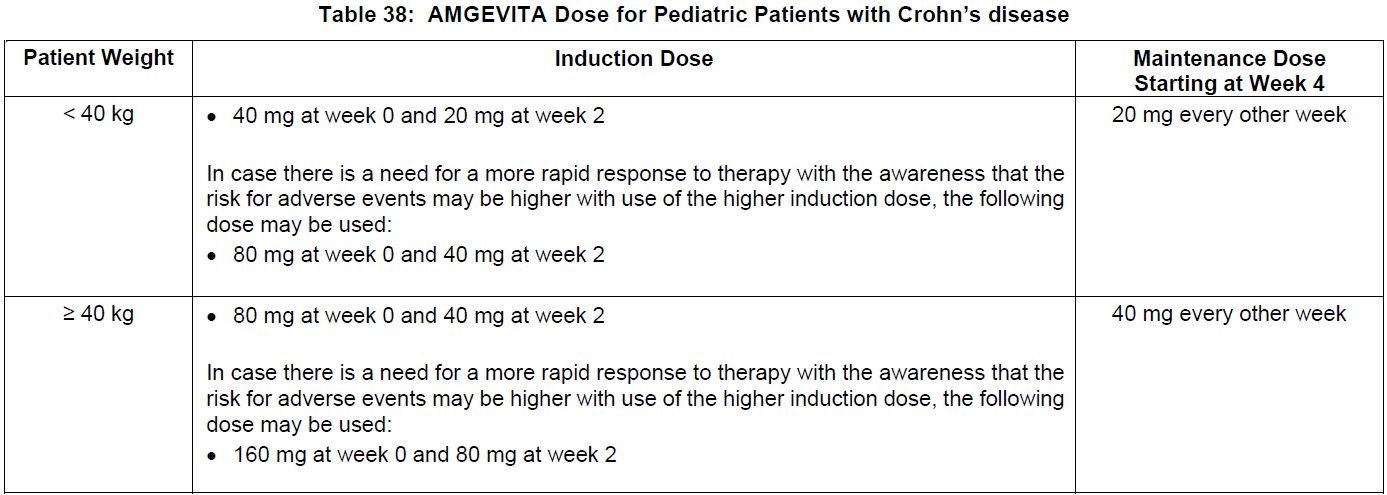 Patients who experience insufficient response may benefit from an increase in dosage: - < 40 kg: 20 mg every week - ≥ 40 kg: 40 mg every week or 80 mg every other week Adalimumab has not been studied in children with Crohn’s disease aged less than 6 years. **Pediatric Plaque Psoriasis** The recommended adalimumab dose for patients from 4 to 17 years of age with plaque psoriasis is based on body weight (see Table 39). Adalimumab is administered via subcutaneous injection. Adalimumab may be available in different strengths and/or presentations. 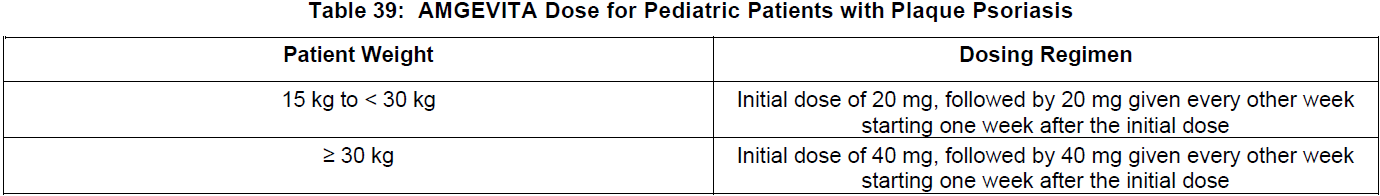 Continued therapy beyond 16 weeks should be carefully considered in a patient not responding within this time period. If retreatment with adalimumab is indicated, the above guidance on dose and treatment duration should be followed. There is no relevant use of adalimumab in children aged less than 4 years in this indication. **Adolescent hidradenitis suppurativa** There are no clinical trials with adalimumab in adolescent patients with hidradenitis suppurativa (HS). The posology of adalimumab in these patients has been determined from pharmacokinetic modeling and simulation. The recommended adalimumab dose in adolescent patients from 12 years of age weighing at least 30 kg with hidradenitis suppurativa is 80 mg at Week 0 followed by 40 mg every other week starting at Week 1 via subcutaneous injection. Adalimumab may be available in other strengths and/or presentations. In adolescent patients with inadequate response to adalimumab 40 mg every other week, an increase in dosage to 40 mg every week or 80 mg every other week may be considered. Antibiotics may be continued during treatment with adalimumab if necessary. It is recommended that the patient should use a topical antiseptic wash on their HS lesions on a daily basis during treatment with adalimumab. Continued therapy beyond 12 weeks should be carefully reconsidered in a patient with no improvement within this time period. Should treatment be interrupted, adalimumab may be re-introduced as appropriate. The benefit and risk of continued long-term treatment should be periodically evaluated. There is no relevant use of adalimumab in children aged less than 12 years in this indication. **Pediatric Uveitis** The recommended dose of adalimumab for pediatric patients 2 years of age and older with chronic non-infectious anterior uveitis is based on body weight (see Table 40). Adalimumab is administered via subcutaneous injection. Adalimumab may be available in different strengths and/or presentations. In paediatric uveitis, there is no experience in the treatment with adalimumab without concomitant treatment with methotrexate.  When adalimumab is initiated, a loading dose of 40 mg for patients < 30 kg or 80 mg for patients ≥ 30 kg may be administered one week prior to the start of maintenance therapy. No clinical data are available on the use of an adalimumab loading dose in children < 6 years of age (see **Pharmacokinetics** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There is no relevant use of adalimumab in children aged less than 2 years in this indication. It is recommended that the benefit and risk of continued long-term treatment should be evaluated on a yearly basis. **Pediatric Ulcerative Colitis** The recommended dose of adalimumab for patients from 5 to 17 years of age with ulcerative colitis is based on body weight (see Table 41). Adalimumab is administered via subcutaneous injection. Adalimumab may be available in different strengths and/or presentations. 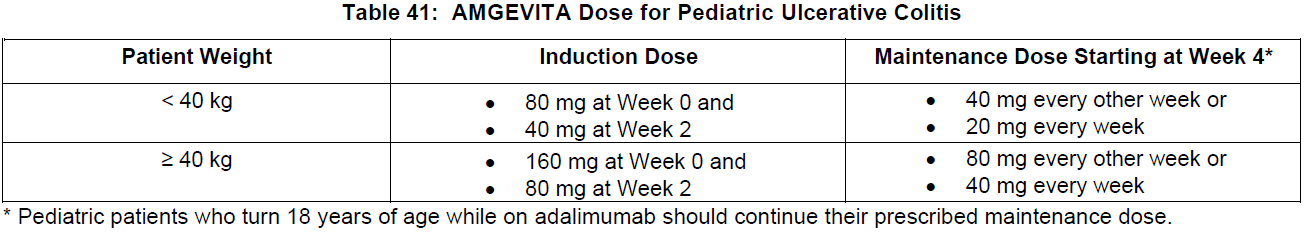 Continued therapy beyond 8 weeks should be carefully considered in patients not showing signs of response within this time period. There is no relevant use of adalimumab in children aged less than 5 years in this indication. **Pediatric Use** Adalimumab has not been studied in children less than 2 years of age and there are limited data on adalimumab treatment in children with weight < 10 kg. The safety and efficacy of adalimumab in pediatric patients for indications other than juvenile idiopathic arthritis (polyarticular juvenile idiopathic arthritis and enthesitis-related arthritis), pediatric Crohn’s disease, pediatric plaque psoriasis, adolescent hidradenitis suppurativa, pediatric uveitis and pediatric ulcerative colitis have not been established. **Geriatric Use** Of the total number of subjects in clinical studies of adalimumab, 9.4% were 65 years and over, while approximately 2.0% were 75 and over. No overall differences in effectiveness were observed between these subjects and younger subjects. No dose adjustment is needed for this population.
SUBCUTANEOUS
Medical Information
**INDICATIONS** **ADULTS** **Rheumatoid Arthritis** AMGEVITA is indicated for reducing signs and symptoms and inhibiting the progression of structural damage and improving physical function in adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more DMARDs. AMGEVITA can be used alone or in combination with methotrexate or other DMARDs. AMGEVITA, in combination with MTX, can also be used in the treatment of patients with recently diagnosed moderate to severely active rheumatoid arthritis who have not received methotrexate. **Psoriatic Arthritis** AMGEVITA is indicated for reducing signs and symptoms of active arthritis in adult patients with moderate to severe psoriatic arthritis when the response to previous DMARD therapy has been inadequate. AMGEVITA has been shown to reduce the rate of progression of peripheral joint damage as measured by X-ray in patients with polyarticular symmetrical subtypes of the disease and to improve physical function. AMGEVITA can be used alone or in combination with DMARDs. **Ankylosing Spondylitis** AMGEVITA is indicated for reducing signs and symptoms in adult patients with active ankylosing spondylitis who have had an inadequate response to conventional therapy. **Crohn’s Disease** AMGEVITA is indicated for the treatment of moderate to severe active Crohn’s disease in adults to reduce the signs and symptoms of the disease and to induce and maintain clinical remission in patients who have had an inadequate response to conventional therapies, or who have lost response to or are intolerant of infliximab. For induction treatment, AMGEVITA should be given in combination with corticosteroids. AMGEVITA can be given as monotherapy in case of intolerance to corticosteroids or when continued treatment with corticosteroids is inadequate. **Ulcerative Colitis** AMGEVITA is indicated for treatment of moderately to severely active ulcerative colitis in adult patients who have had an inadequate response to conventional therapy including corticosteroids and/or 6-mercaptopurine (6-MP) or azathioprine (AZA), or who are intolerant to or have medical contraindications for such therapies. **Plaque Psoriasis** AMGEVITA is indicated for the treatment of adult patients with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy and when other systemic therapies are medically less appropriate. **Hidradenitis Suppurativa** AMGEVITA is indicated for the treatment of active moderate to severe hidradenitis suppurativa (acne inversa) in adult patients with an inadequate response to conventional systemic HS therapy. **Uveitis** AMGEVITA is indicated for the treatment of non-infectious intermediate, posterior and panuveitis in adult patients who have had an inadequate response to corticosteroids, in patients in need of corticosteroid-sparing, or in whom corticosteroid treatment is inappropriate. **PEDIATRICS** **Juvenile Idiopathic Arthritis** **_Polyarticular Juvenile Idiopathic Arthritis_** AMGEVITA in combination with methotrexate is indicated for the treatment of active polyarticular juvenile idiopathic arthritis (pJIA), in patients 2 years of age and older, who have had an inadequate response to one or more disease-modifying anti-rheumatic drugs (DMARDS). AMGEVITA can be given as monotherapy in case of intolerance to methotrexate or when continued treatment with methotrexate is inappropriate (for the efficacy in monotherapy see **CLINICAL STUDIES** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). AMGEVITA has not been studied in patients aged less than 2 years. _**Enthesitis-Related Arthritis**_ AMGEVITA is indicated for the treatment of active enthesitis-related arthritis in patients, 6 years of age and older, who have had an inadequate response to, or who are intolerant of, conventional therapy. **Pediatric Crohn’s Disease** AMGEVITA is indicated for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients, 6 years of age and older, with moderately to severely active Crohn’s disease who have had an inadequate response to conventional therapy. **Pediatric Plaque Psoriasis** AMGEVITA is indicated for the treatment of severe chronic plaque psoriasis in children and adolescents from 4 years of age who have had an inadequate response to or are inappropriate candidates for topical therapy and phototherapy. **Adolescent Hidradenitis Suppurativa** AMGEVITA is indicated for the treatment of active moderate to severe hidradenitis suppurativa (acne inversa) in adolescents from 12 years of age with an inadequate response to conventional systemic hidradenitis suppurativa (HS) therapy. **Pediatric Uveitis** AMGEVITA is indicated for the treatment of chronic non-infectious anterior uveitis in pediatric patients 2 years of age and older who have had an inadequate response to or are intolerant to conventional therapy, or in whom conventional therapy is inappropriate. **Pediatric Ulcerative Colitis** AMGEVITA is indicated for inducing and maintaining clinical remission in pediatric patients 5 years of age or older with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy including corticosteroids and/or 6-mercaptopurine (6-MP) or azathioprine (AZA), or who are intolerant to or have medical contraindications for such therapies.
**CONTRAINDICATIONS** AMGEVITA should not be administered to patients with known hypersensitivity to AMGEVITA or any of its excipients. Active tuberculosis or other severe infections such as sepsis, abscesses, and opportunistic infections (see **WARNINGS AND PRECAUTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Moderate to severe heart failure (NYHA class III/IV) (see **WARNINGS AND PRECAUTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L04AB04
adalimumab
Manufacturer Information
AMGEN BIOTECHNOLOGY SINGAPORE PTE. LTD.
Amgen Manufacturing Limited
Active Ingredients
Documents
Package Inserts
Amgevita IFU PFS.pdf
Approved: February 25, 2021
