Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Posology Non-Small Cell Lung Cancer (NSCLC): _Monotherapy:_ The recommended dose of gemcitabine is 1,000 mg/m2, given by 30-minute intravenous infusion. This should be repeated once weekly for 3 weeks, followed by a 1 week rest period. This 4-week cycle is then repeated. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. _Combination use:_ Gemcitabine in combination with cisplatin has been investigated using two dosing regimen. One regimen used a 3-week schedule and the other used a 4-week schedule. The 3-week schedule used gemcitabine 1,250 mg/m2, given by 30-minute intravenous infusion, on Days 1 and 8 of each 21-day cycle. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. The 4-week schedule used gemcitabine 1,000 mg/m2, given by 30-minute intravenous infusion, on Days 1, 8 and 15 of each 28-day cycle. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. Cisplatin has been used at doses between 75–100 mg/m2 once every 3 or 4 weeks. Pancreatic Cancer Monotherapy: The recommended dose of gemcitabine is 1,000 mg/m2, given by 30-minute intravenous infusion. This should be repeated once weekly for up to 7 weeks, followed by a week of rest. Subsequent cycles should consist of injections once weekly for 3 consecutive weeks out of every 4 weeks. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. Bladder Cancer Combination use: The recommended dose for gemcitabine is 1,000 mg/m2, given by 30- minute infusion. The dose should be given on Days 1, 8 and 15 of each 28-day cycle in combination with cisplatin. Cisplatin is given at a recommended dose of 70 mg/m2 on Day 1 following gemcitabine or Day 2 of each 28-day cycle. This 4-week cycle is then repeated. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. A clinical trial showed more myelosuppression when cisplatin was used in doses of 100 mg/m2. Breast Cancer Combination use: Gemcitabine in combination with paclitaxel is recommended using paclitaxel 175 mg/m2 administered on Day 1 over approximately 3 hours as an intravenous infusion, followed by gemcitabine 1,250 mg/m2 as a 30-minute intravenous infusion on Days 1 and 8 of each 21-day cycle. Dose reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. Monitoring for toxicity and dose modification due to toxicity _Dose modification due to non-haematological toxicity_ Periodic physical examination and checks of renal and hepatic function should be made to detect non-haematological toxicity. Dosage reduction with each cycle or within a cycle may be applied based upon the grade of toxicity experienced by the patient. In general, for severe (Grade 3 or 4) non-haematological toxicity, except nausea/vomiting, therapy with gemcitabine should be withheld or decreased depending on the judgement of the treating physician. Doses should be withheld until toxicity has resolved in the opinion of the physician. For cisplatin and paclitaxel dosage adjustment in combination therapy, please refer to the corresponding manufacturers’ prescribing information. _Dose modification due to haematological toxicity_ _Initiation of a cycle_ For all indications, the patient must be monitored before each dose for platelet and granulocyte counts. Patients should have an absolute granulocyte count of at least 1,500 (x 106/l) and platelet count of 100,000 (x 106/l) prior to the initiation of a cycle. _Within a cycle_ Dose modifications of gemcitabine within a cycle should be performed according to the following tables: 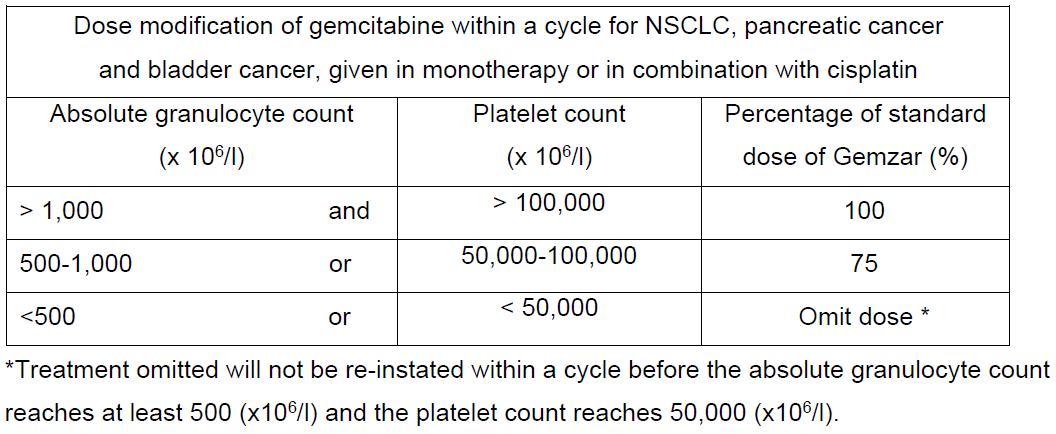 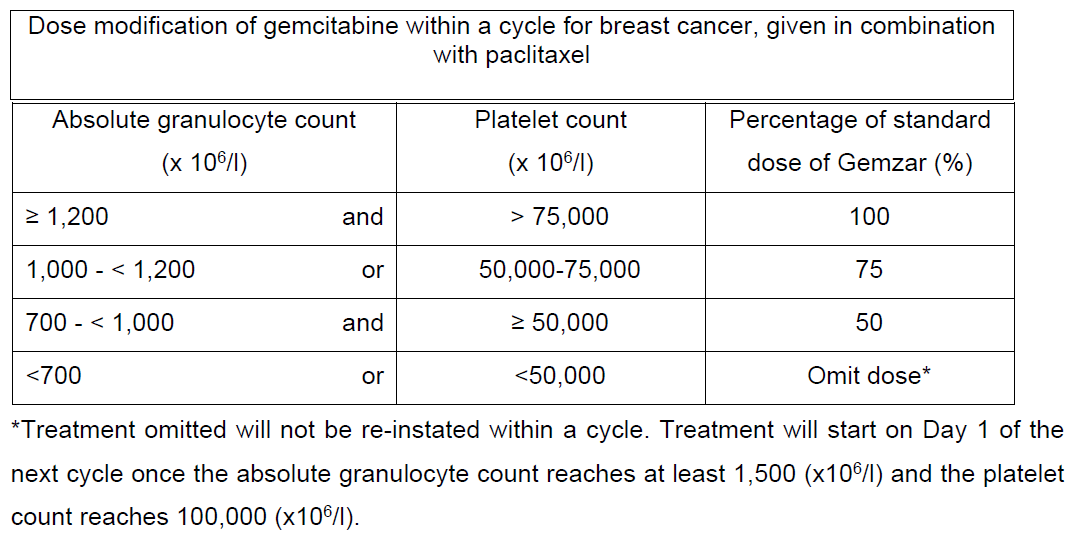 _Dose modifications due to haematological toxicity in subsequent cycles, for all indications_ The gemcitabine dose should be reduced to 75% of the original cycle initiation dose, in the case of the following haematological toxicities: - Absolute granulocyte count < 500 x 106/l for more than 5 days - Absolute granulocyte count < 100 x 106/l for more than 3 days - Febrile neutropaenia - Platelets < 25,000 x 106/l - Cycle delay of more than 1 week due to toxicity Special populations _Patients with renal or hepatic impairment_ Gemcitabine should be used with caution in patients with hepatic or renal impairment as there is insufficient information from clinical studies to allow for clear dose recommendations for these patient populations (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dose reduction is recommended in patients with elevated serum bilirubin concentration because such patients are at increased risk of toxicity. The dose modifications are based on a Phase 1 study of cancer patients with elevated serum bilirubin concentrations (median 50 micromole/L, range 30–100 micromole/L,) who were administered gemcitabine monotherapy, 8 out of 10 patients experienced toxicity at a gemcitabine dose of 950 mg/m2 compared with 3 out of 8 at 800 mg/m2. The toxicity was mostly related to the liver. In the same study, patients with elevated serum creatinine concentration appeared to experience increased sensitivity to gemcitabine. However, the data based on 15 patients was not sufficient to make dosing recommendation. _Elderly patients (> 65 years)_ Gemcitabine has been well tolerated in patients over the age of 65. There is no evidence to suggest that dose adjustments, other than those already recommended for all patients, are necessary in elderly, although gemcitabine clearance and half-life are affected by age (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population (< 18 years)_ Gemcitabine is not recommended for use in children under 18 years of age due to insufficient data on safety and efficacy. Method of administration Gemzar is tolerated well during infusion and may be administered ambulant. If extravasation occurs, generally the infusion must be stopped immediately and started again in another blood vessel. The patient should be monitored carefully after the administration. For instructions on reconstitution, see section 6.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Non-Small Cell Lung Cancer (NSCLC) Gemcitabine, in combination with cisplatin, is indicated as a first line treatment of patients with locally advanced (inoperable Stage IIIA or IIIB) or metastatic (Stage IV) NSCLC. Gemcitabine is indicated for the palliative treatment of adult patients with locally advanced or metastatic NSCLC. Pancreatic cancer Gemcitabine is indicated for the treatment of adult patients with locally advanced or metastatic adenocarcinoma of the pancreas. Gemcitabine is indicated for patients with 5- FU refractory pancreatic cancer. Bladder cancer Gemcitabine is indicated for the treatment of advanced bladder cancer (muscle invasive Stage IV tumours with or without metastases) in combination with cisplatin therapy. Breast Cancer Gemcitabine, in combination with paclitaxel, is indicated for the treatment of patients with unresectable, locally recurrent or metastatic breast cancer who have relapsed following adjuvant/neoadjuvant chemotherapy. Prior chemotherapy should have included an anthracycline unless clinically contraindicated.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients. Breast-feeding (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01BC05
gemcitabine
Manufacturer Information
DKSH SINGAPORE PTE. LTD.
Vianex S.A. - Plant C (Bulk Manufacturer and Primary Packager)
Active Ingredients
Documents
Package Inserts
Gemzar Powder for Solution for Infusion PI.pdf
Approved: May 30, 2019
