Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**2.2 DOSAGE AND ADMINISTRATION** **General** _**Patient Selection**_ _Solid Tumors_ A validated assay is required for the selection of patients with _NTRK_ fusion-positive locally advanced or metastatic solid tumors. _NTRK_ fusion-positive status should be established prior to initiation of Rozlytrek therapy. _NSCLC_ A validated assay is required for the selection of patients with _ROS1_-positive locally advanced or metastatic NSCLC. _ROS1_-positive status should be established prior to initiation of Rozlytrek therapy. **Dosage** Rozlytrek hard capsules can be taken with or without food, swallowed whole and must not be opened or dissolved. _Adults_ The recommended dose of Rozlytrek for adults is 600 mg given orally, once daily (see section _3.2 Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Pediatric patients 12 years and older_ The recommended dose of Rozlytrek for pediatric patients, 12 years and older, who have the ability to swallow capsules is 300 mg/m2 orally, once daily (see Table 1). (See section _3.2 Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 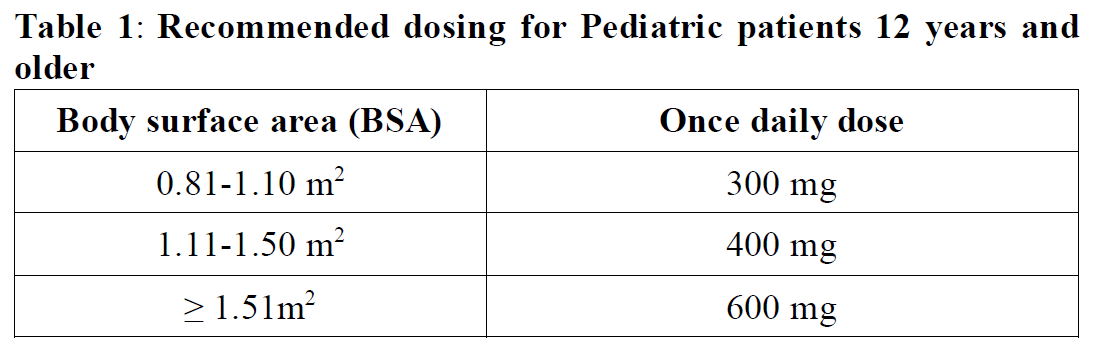 **Duration of Treatment** It is recommended that patients are treated with Rozlytrek until disease progression or unacceptable toxicity. **Delayed or Missed Doses** If a planned dose of Rozlytrek is missed, patients can make up that dose unless the next dose is due within 12 hours. If vomiting occurs immediately after taking a dose of Rozlytrek, patients may repeat that dose. **Dose Modifications** Management of adverse events may require temporary interruption, dose reduction, or discontinuation of treatment with Rozlytrek, based on the prescriber’s assessment of the patient’s safety or tolerability. _Adults_ For adults, the dose of Rozlytrek may be reduced up to 2 times, based on tolerability. Table 2 provides general dose reduction advice for adult patients. Rozlytrek treatment should be permanently discontinued if patients are unable to tolerate a dose of 200 mg once daily. 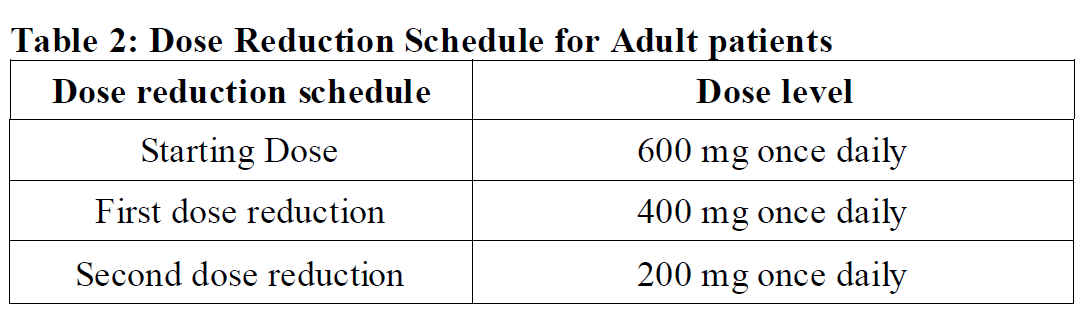 _Pediatric Patients_ Table 3 provides specific dose reduction advice for pediatric patients. For pediatric patients, the dose of Rozlytrek may be reduced up to 2 times, based on tolerability. For some patients an intermittent dosing schedule is required to achieve the recommended reduced total weekly pediatric dose. Rozlytrek treatment should be permanently discontinued if patients are unable to tolerate the lowest reduced dose. 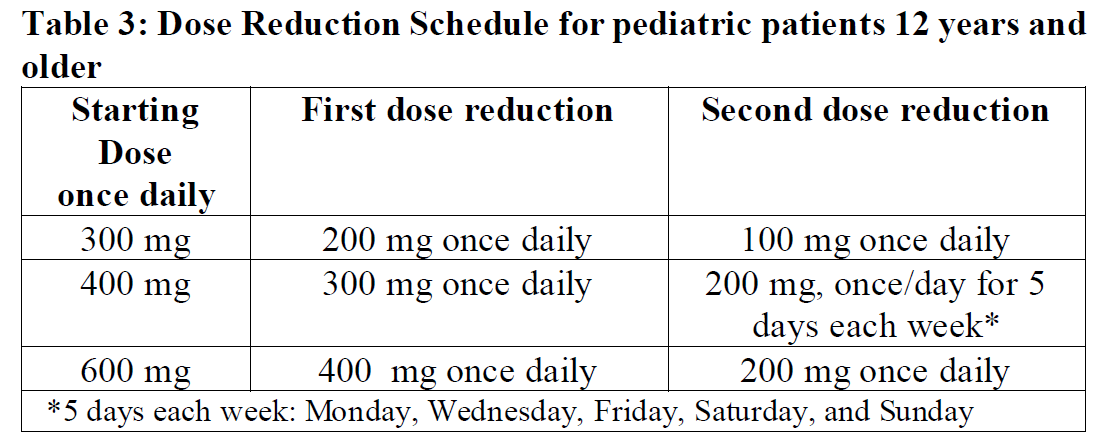 **Dose Modifications for Specific Adverse Reactions** Recommendations for Rozlytrek dose modifications for adults and pediatric patients for specific adverse reactions are provided in Table 4. (See section _2.4.1 Warnings and Precautions_ and section _2.6 Undesirable Effects_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 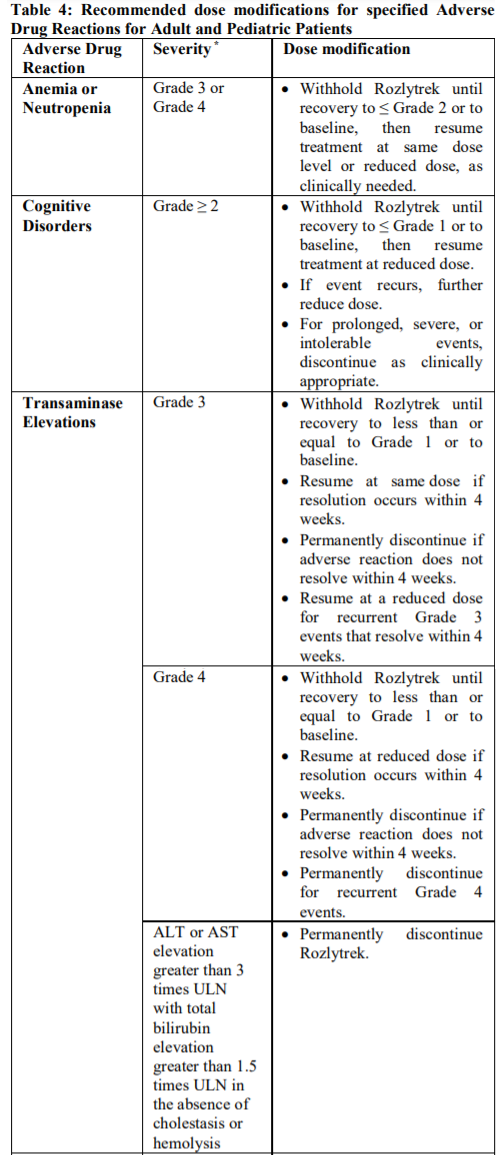 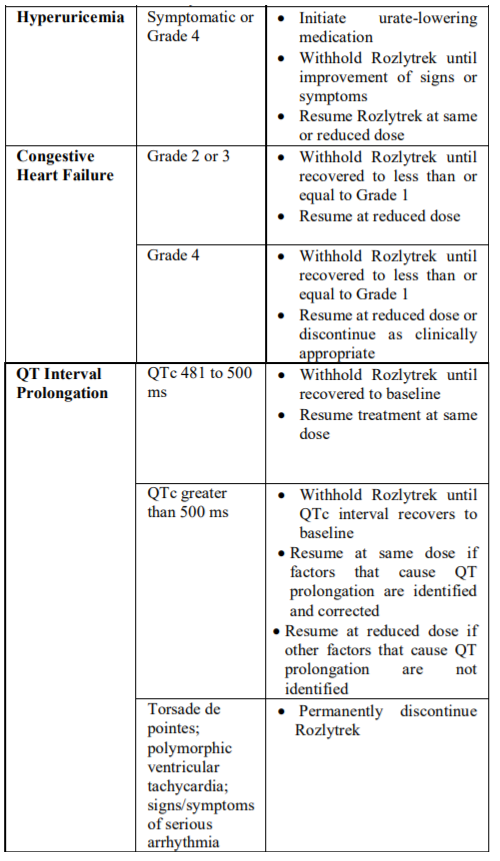 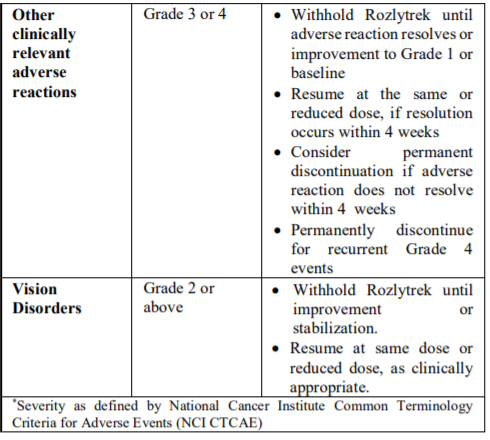 **Dose Modifications for Specific Drug Interactions** **_Concomitant strong or moderate CYP3A inhibitors:_** _Adults_ The concomitant use of strong or moderate CYP3A inhibitors and Rozlytrek in adults should be avoided or limited to 14 days or less. If concomitant use of strong or moderate CYP3A inhibitors cannot be avoided, Rozlytrek dose should be reduced to 100 mg once daily for use with strong CYP3A inhibitors and to 200 mg once daily for use with moderate CYP3A inhibitors. After discontinuation of the concomitant strong or moderate CYP3A inhibitors, Rozlytrek dose that was taken prior to initiating the strong or moderate CYP3A inhibitor can be resumed. A wash out period may be required for CYP3A4 inhibitors with long half-life. (See section _2.8 Interactions with Other Medicinal Products and other forms of Interaction_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Pediatric patients_ The concomitant use of strong or moderate CYP3A inhibitors in pediatric patients should be avoided. (See section _2.8 Interactions with Other Medicinal Products and other forms of Interaction_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Concomitant CYP3A inducers:**_ Co-administration of Rozlytrek with CYP3A inducers in adult and pediatric patients should be avoided. (See section _2.8 Interactions with Other Medicinal Products and other forms of Interaction_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **2.2.1 Special Dosage Instructions** **Pediatric use** Pediatric patients must have the ability to swallow whole Rozlytrek capsules. Dosage for patients 12 years and older is based on body surface area (mg/m2) with a maximum daily dose of 600 mg (see Table 1 for pediatric dosing). The safety and efficacy of Rozlytrek in children below 12 years of age have not been established. **Geriatric use** No dose adjustment of Rozlytrek is required in patients ≥ 65 years of age. (See section _3.2.5 Pharmacokinetics in Special Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal Impairment** No dose adjustment is required in patients with mild or moderate renal impairment. The safety and efficacy of Rozlytrek have not been studied in patients with severe renal impairment. (See sections _2.5 Use in Special Populations_ and section _3.2.5 Pharmacokinetics in Special Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic Impairment** No dose adjustment is required in patients with underlying mild, moderate or severe hepatic impairment based on a study in subjects with hepatic impairment. (See section _2.5 Use in Special Populations_ and section _3.2.5 Pharmacokinetics in Special Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with severe hepatic impairment should be carefully monitored for hepatic function and adverse reactions (see Table 4). **Other Special Patient Populations** _Ethnicity_ No dose adjustment is necessary for patients of different ethnicities (see section _3.2.5 Pharmacokinetics in Special Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**2.1 THERAPEUTIC INDICATION(S)** **_Solid tumors_** Rozlytrek is indicated for the treatment of adult and pediatric patients 12 years of age and older, with neurotrophic tyrosine receptor kinase ( _NTRK_) fusion-positive solid tumors without a known acquired resistance mutation, that are locally advanced, metastatic or where surgical resection is likely to result in severe morbidity, and who have progressed following prior therapies or have no satisfactory alternative treatments. **_Non-small cell lung cancer (NSCLC)_** Rozlytrek is indicated for the treatment of adult patients with _ROS1_-positive, locally advanced or metastatic NSCLC.
**2.3 CONTRAINDICATIONS** Rozlytrek is contraindicated in patients with a known hypersensitivity to entrectinib or any of the excipients.
L01XE56
xl 01 xe 56
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Mayne Pharma Inc.
F. Hoffmann-La Roche Ltd (Primary and secondary packaging)
Active Ingredients
Documents
Package Inserts
Rozlytrek Hard Capsule PI.pdf
Approved: March 15, 2023
