Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Posology The recommended durations of treatment are 5–14 days for cSSTI and 5–7 days for CAP. 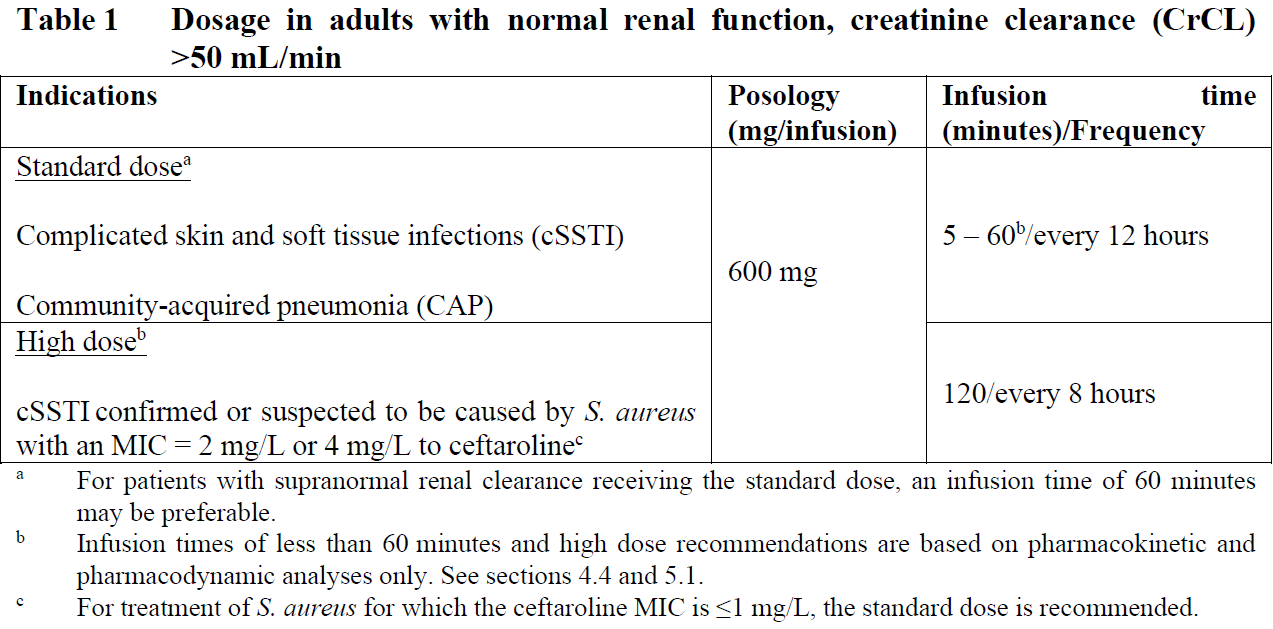 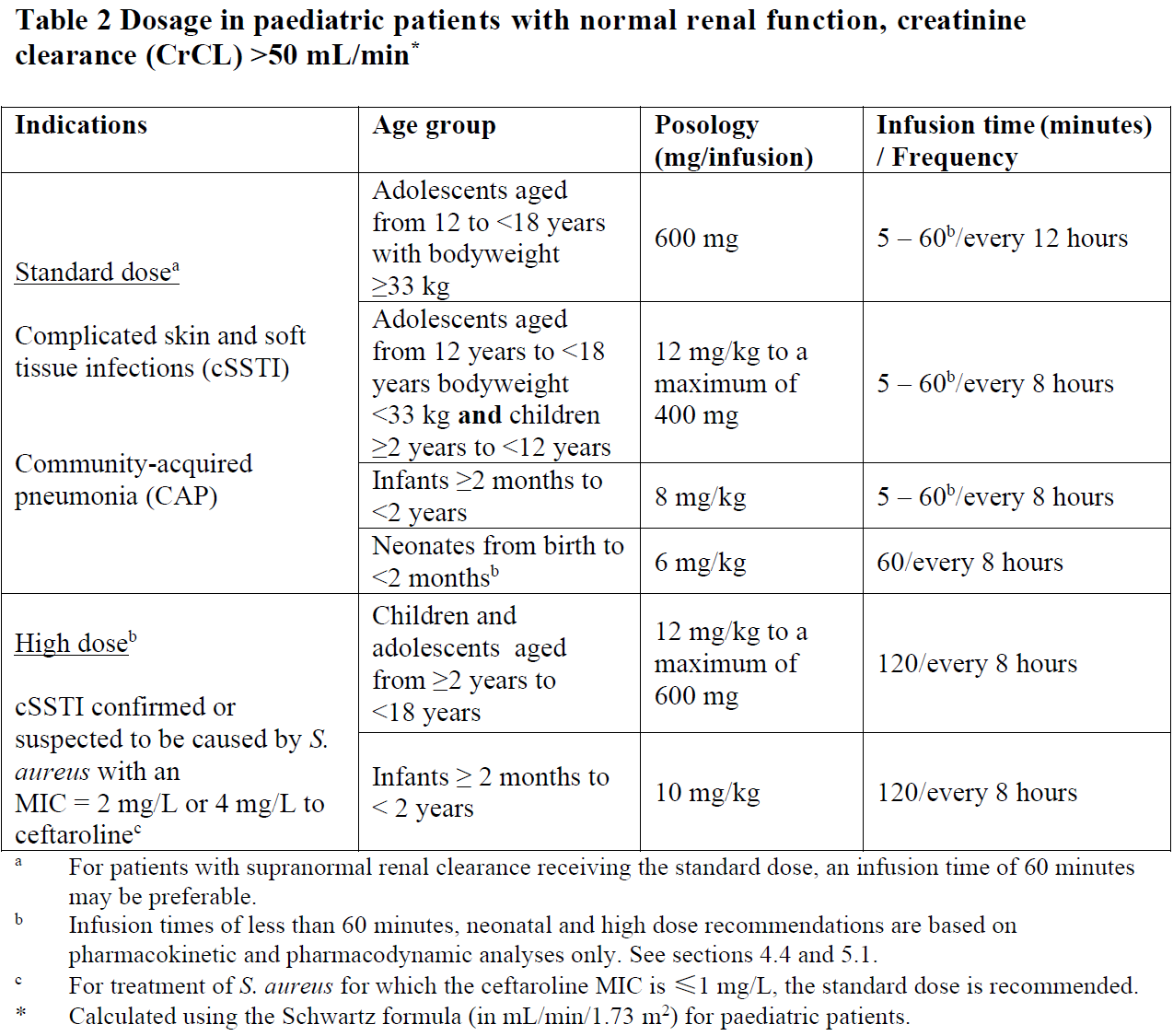 Special populations _Elderly_ No dosage adjustment is required for the elderly with creatinine clearance values >50 mL/min (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ The dose should be adjusted when creatinine clearance (CrCL) is ≤50 mL/min, as shown in Tables 3 and 4 (see sections 4.9 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended durations of treatment are 5–14 days for cSSTI and 5–7 days for CAP. 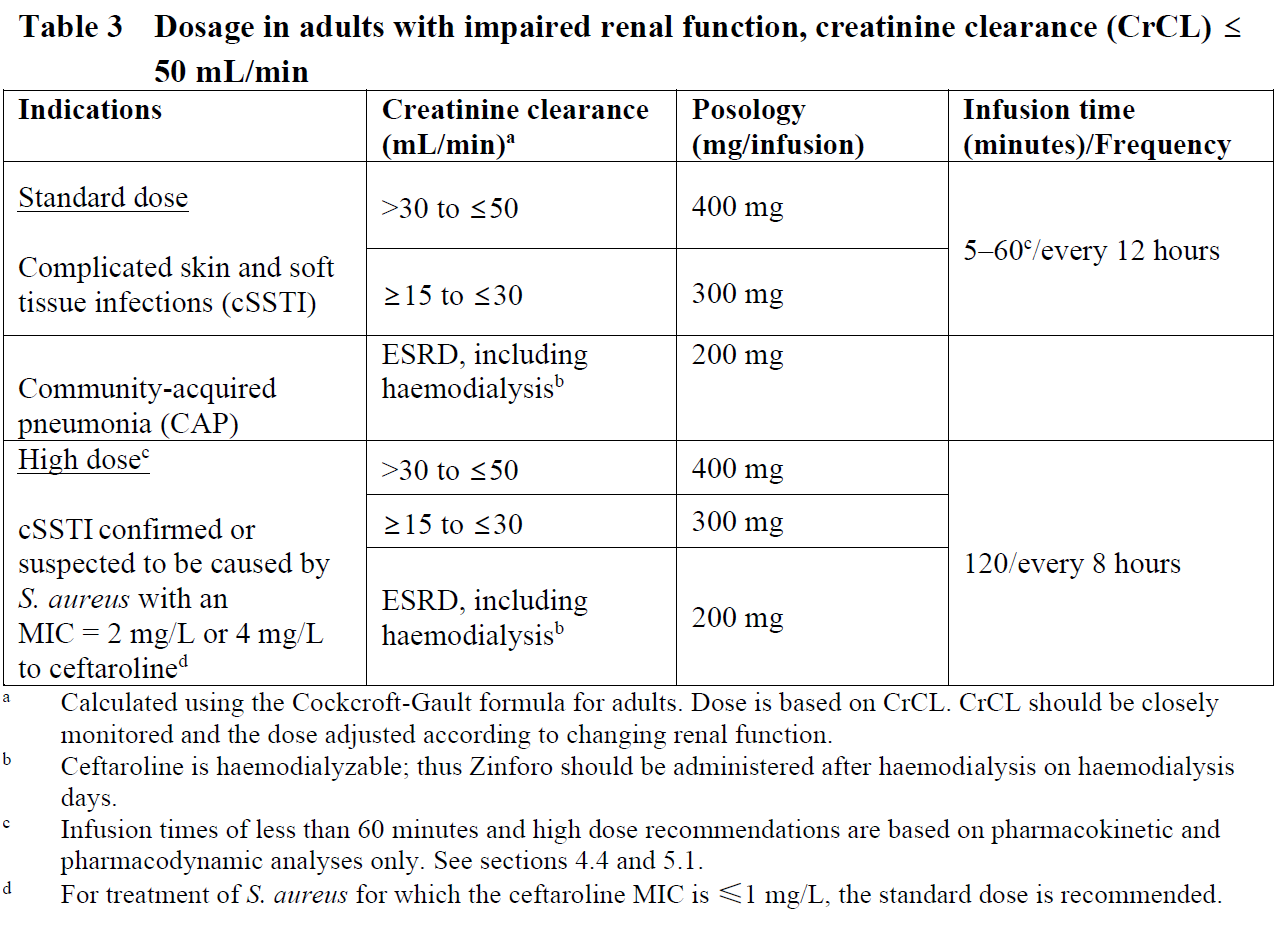 Dose recommendations for neonates, infants and children and adolescents are based on pharmacokinetic (PK) modelling. There is insufficient information to recommend dosage adjustments in adolescents aged from 12 to <18 years with bodyweight <33 kg and in children aged from 2 to 12 years with End-stage renal disease (ESRD). There is insufficient information to recommend dosage adjustments in paediatric patients <2 years with moderate or severe renal impairment or ESRD. 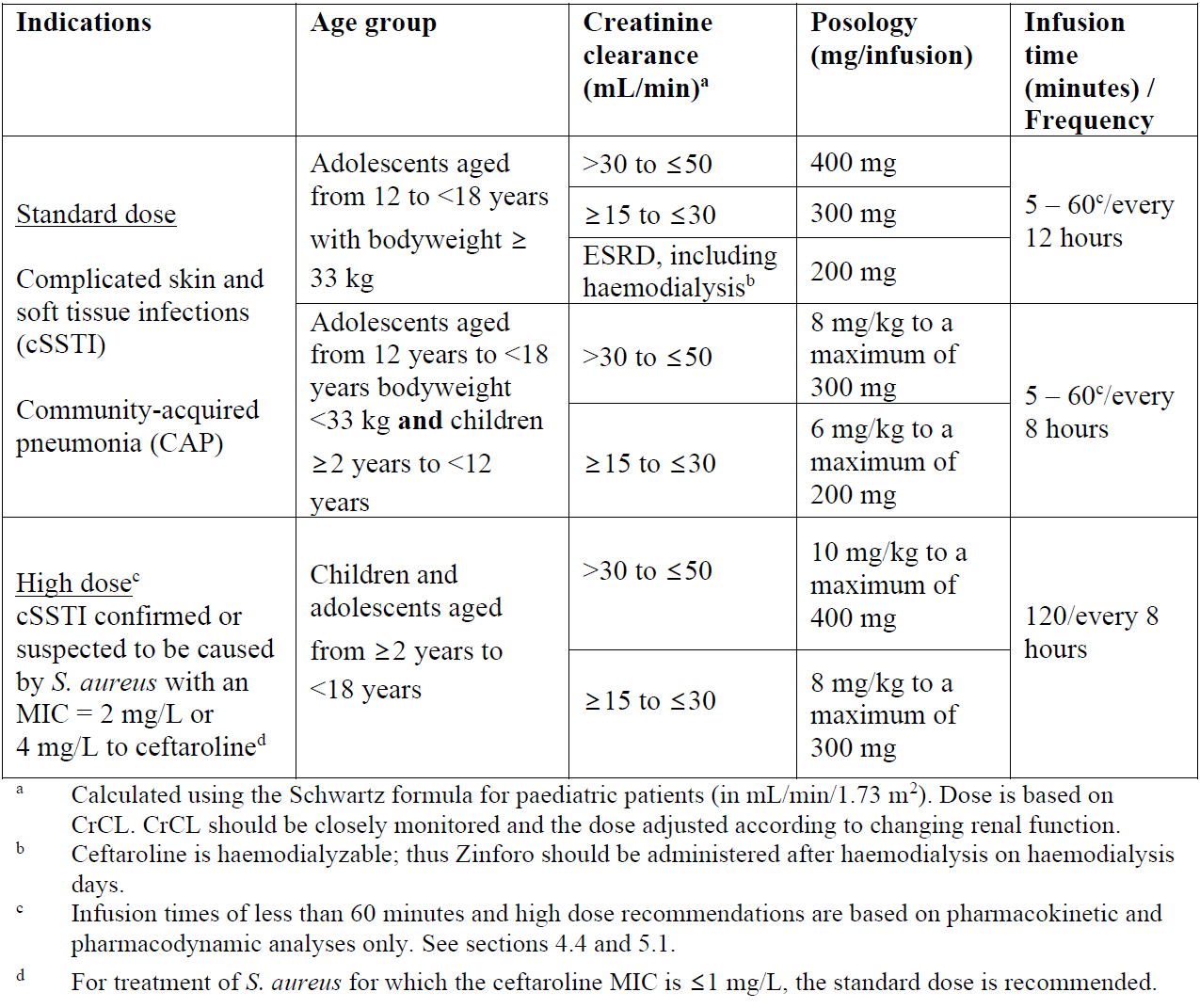 _Hepatic impairment_ No dosage adjustment is considered necessary in patients with hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of administration Intravenous use. Zinforo is administered by intravenous infusion over 5 to 60 minutes for standard dose or 120 minutes for high dose (for cSSTI caused by S. aureus with MIC of 2 or 4 mg/L to ceftaroline) in infusion volumes of 50 mL, 100 mL or 250 mL (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Infusion related reactions (such as phlebitis) can be managed by prolonging the infusion duration. Infusion volumes for paediatric patients will vary according to the weight of the child. The infusion solution concentration during preparation and administration should not exceed 12 mg/mL ceftaroline fosamil. For instructions on reconstitution and dilution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Zinforo is indicated for the treatment of the following infections caused by susceptible isolates of the designated microorganisms: - Complicated skin and soft tissue infections (cSSTI) - Community-acquired pneumonia (CAP) Zinforo is indicated in neonates, infants, children, adolescents and adults.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of its excipients. Hypersensitivity to the cephalosporin class of antibacterials. Immediate and severe hypersensitivity (e.g. anaphylactic reaction) to any other type of beta- lactam antibacterial agent (e.g. penicillins or carbapenems).
J01DI02
ceftaroline fosamil
Manufacturer Information
PFIZER PRIVATE LIMITED
ACS Dobfar S.p.A.
ACS Dobfar S.p.A. (intermediate – sterile bulk drug product blend)
Active Ingredients
Documents
Package Inserts
Zinforo 600mg-vial PI.pdf
Approved: December 1, 2022
