Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**IV. DOSAGE AND ADMINISTRATION** The usual dose of INVANZ in patients 13 years of age and older is 1 gram (g) given once a day. The usual dose of INVANZ in patients 3 months to 12 years of age is 15 mg/kg twice daily (not to exceed 1 g/day). INVANZ is not recommended in children under 3 months of age, as no data are available. INVANZ may be administered by intravenous (IV) infusion or intramuscular (IM) injection. When administered intravenously, INVANZ should be infused over a period of 30 minutes. Intramuscular administration of INVANZ may be used as an alternative to intravenous administration in the treatment of those infections for which intramuscular therapy is appropriate. The usual duration of therapy with INVANZ is 3 to 14 days but varies by the type of infection and causative pathogen(s). (See INDICATIONS.) When clinically indicated, a switch to an appropriate oral antimicrobial may be implemented if clinical improvement has been observed. In controlled clinical studies, patients were treated from 3 to 14 days. Total treatment duration was determined by the treating physician based on site and severity of the infection, and on the patient’s clinical response. In some studies, treatment was converted to oral therapy at the discretion of the treating physician after clinical improvement had been demonstrated. _Prophylaxis of surgical site infection following elective colorectal surgery:_ To prevent surgical site infections following elective colorectal surgery in adults, the recommended dosage is 1 g IV administered as a single intravenous dose given 1 hour prior to the surgical incision. _Patients with renal insufficiency:_ INVANZ may be used for the treatment of infections in adult patients with renal insufficiency. In patients whose creatinine clearance is >30 mL/min/1.73 m2, no dosage adjustment is necessary. Adult patients with advanced renal insufficiency (creatinine clearance ≤30 mL/min/1.73 m2), including those on hemodialysis, should receive 500 mg daily. There are no data in pediatric patients with renal insufficiency. _Patients on Hemodialysis:_ In a clinical study, following a single 1 g IV dose of ertapenem given immediately prior to a hemodialysis session, approximately 30% of the dose was recovered in the dialysate. When adult patients on hemodialysis are given the recommended daily dose of 500 mg of INVANZ within 6 hours prior to hemodialysis, a supplementary dose of 150 mg is recommended following the hemodialysis session. If INVANZ is given at least 6 hours prior to hemodialysis, no supplementary dose is needed. There are no data in patients undergoing peritoneal dialysis or hemofiltration. There are no data in pediatric patients on hemodialysis. When only the serum creatinine is available, the following formula\*\* may be used to estimate creatinine clearance. The serum creatinine should represent a steady state of renal function. 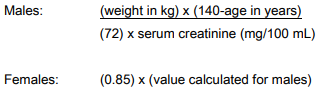 No dosage adjustment is recommended in patients with impaired hepatic function (see CLINICAL PHARMACOLOGY, Characteristics in Patients, Hepatic Insufficiency – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **INSTRUCTIONS FOR USE** _**Patients 13 years of age and older**_ _Preparation for intravenous administration:_ DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). **INVANZ MUST BE RECONSTITUTED AND THEN DILUTED PRIOR TO ADMINISTRATION.** 1. Reconstitute the contents of a 1 g vial of INVANZ with 10 mL of one of the following: Water for Injection, 0.9% Sodium Chloride Injection or Bacteriostatic Water for Injection. 2. Shake well to dissolve and immediately transfer contents of the reconstituted vial to 50 mL of 0.9% Sodium Chloride Injection. 3. Complete the infusion within 6 hours of reconstitution. _Preparation for intramuscular administration:_ **INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION.** 1. Reconstitute the contents of a 1 g vial of INVANZ with 3.2 mL of 1.0% or 2.0% lidocaine HCl injection\*\*\* ( **without epinephrine**). Shake vial thoroughly to form solution. 2. Immediately withdraw the contents of the vial and administer by deep intramuscular injection into a large muscle mass (such as the gluteal muscles or lateral part of the thigh). 3. The reconstituted IM solution should be used within 1 hour after preparation. **Note: The reconstituted solution should not be administered intravenously.** _**Pediatric patients 3 months to 12 years of age**_ _Preparation for intravenous administration:_ DO NOT MIX OR CO-INFUSE INVANZ WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). **INVANZ MUST BE RECONSTITUTED AND THEN DILUTED PRIOR TO ADMINISTRATION.** 1. Reconstitute the contents of a 1 g vial of INVANZ with 10 mL of one of the following: Water for Injection, 0.9% Sodium Chloride Injection or Bacteriostatic Water for Injection. 2. Shake well to dissolve and immediately withdraw a volume equal to 15 mg/kg of body weight (not to exceed 1 g/day) and dilute in 0.9% Sodium Chloride Injection to a final concentration of 20 mg/mL or less. 3. Complete the infusion within 6 hours of reconstitution. _Preparation for intramuscular administration:_ **INVANZ MUST BE RECONSTITUTED PRIOR TO ADMINISTRATION.** 1. Reconstitute the contents of a 1 g vial of INVANZ with 3.2 mL of 1.0% or 2.0% lidocaine HCl injection\*\*\* ( **without epinephrine**). Shake vial thoroughly to form solution. 2. Immediately withdraw a volume equal to 15 mg/kg of body weight (not to exceed 1 g/day) and administer by deep intramuscular injection into a large muscle mass (such as the gluteal muscles or lateral part of the thigh). 3. The reconstituted IM solution should be used within 1 hour after preparation. **Note: The reconstituted solution should not be administered intravenously.** Parenteral drug products should be inspected visually for particulate matter and discoloration prior to use, whenever solution and container permit. Solutions of INVANZ range from colorless to pale yellow. Variations of color within this range do not affect the potency of the product. * * * \\*\\* Cockcroft and Gault equation: Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976. \\*\\*\\* Refer to the prescribing information for lidocaine HCl.
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**III. INDICATIONS** Treatment INVANZ is indicated for the treatment of patients with moderate to severe infections caused by susceptible strains of microorganisms, as well as initial empiric therapy prior to the identification of causative organisms in the infections listed below: - _Complicated Intra-Abdominal Infections_ - _Complicated Skin and Skin Structure Infections including diabetic lower extremity infections_ - _Community Acquired Pneumonia_ - _Complicated Urinary Tract Infections including pyelonephritis_ - _Acute Pelvic Infections including postpartum endomyometritis, septic abortion and post surgical gynecologic infections_ To reduce the development of drug-resistant bacteria and maintain effectiveness of INVANZ and other antibacterial drugs, INVANZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Prevention INVANZ is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery.
**VI. CONTRAINDICATIONS** INVANZ is contraindicated in patients with known hypersensitivity to any component of this product or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to beta-lactams. Due to the use of lidocaine HCl as a diluent, INVANZ administered intramuscularly is contraindicated in patients with a known hypersensitivity to local anesthetics of the amide type and in patients with severe shock or heart block. (Refer to the prescribing information for lidocaine HCl.)
J01DH03
ertapenem
Manufacturer Information
MSD PHARMA (SINGAPORE) PTE. LTD.
FAREVA Mirabel
Active Ingredients
Documents
Package Inserts
Invanz PI_Approved.pdf
Approved: July 28, 2022
