Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4 Dosage regimen and administration** **Patient selection** Patients should be selected for treatment with Tabrecta based on the presence of a MET exon 14 skipping mutation in tumor or plasma specimens using a validated test. If a MET exon 14 skipping mutation is not detected in a plasma specimen, tumor tissue should be tested if feasible. **Dosage regimen** **General target population** The recommended dose of Tabrecta is 400 mg orally twice daily with or without food (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Treatment duration** Treatment should be continued based on individual safety and tolerability and as long as the patient is deriving clinical benefit from therapy. **Dose modifications for adverse drug reactions** The recommended dose reduction schedule for the management of adverse drug reactions (ADRs) based on individual safety and tolerability is listed in Table 4-1. 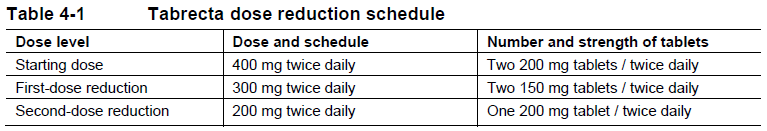 Tabrecta should be permanently discontinued in patients unable to tolerate 200 mg orally twice daily. Recommendations for dose modifications of Tabrecta for ADRs are provided in Table 4-2. 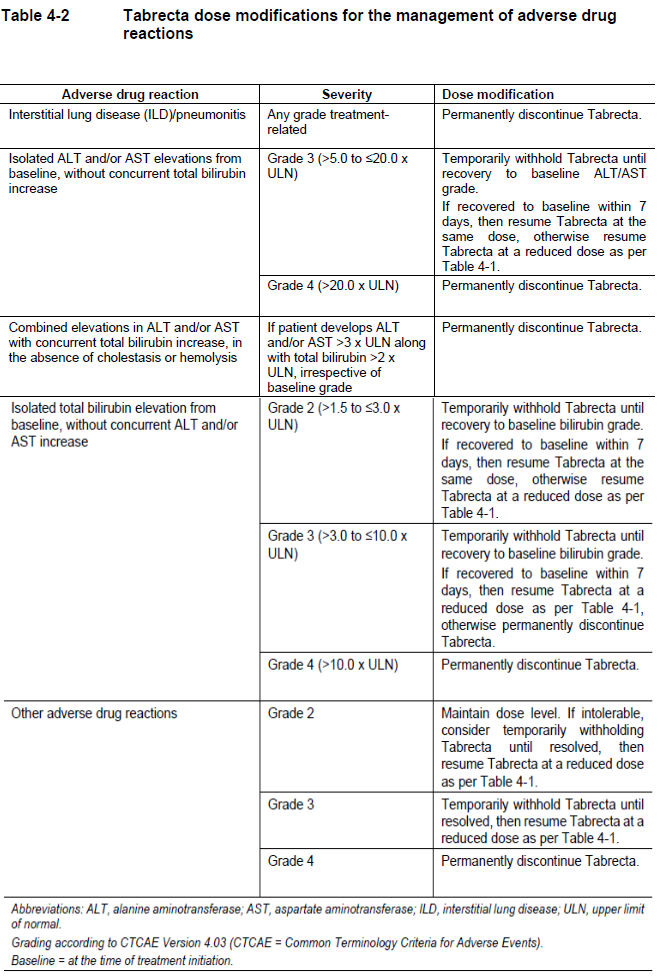 **Special populations** **Renal impairment** No dose adjustment is necessary in patients with mild or moderate renal impairment based on population pharmacokinetic evaluations; Tabrecta has not been studied in patients with severe renal impairment (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** No dose adjustment is necessary in patients with mild, moderate, or severe hepatic impairment (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Pediatric patients (below 18 years of age)** The safety and efficacy of Tabrecta in pediatric patients have not been established. **Geriatric patients (65 years of age or older)** No dose adjustment is necessary in patients 65 years of age or older. **Method of administration** Tabrecta should be taken orally twice daily with or without food. The tablets should be swallowed whole and should not be broken, chewed, or crushed. If a dose of Tabrecta is missed or vomiting occurs, the patient should not make up the dose, but take the next dose at the scheduled time.
ORAL
Medical Information
**3 Indications** Tabrecta is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) with a MET exon 14 skipping mutation.
**5 Contraindications** None.
pending
xpending
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Lek d.d. (Primary and Secondary Packager)
Novartis Pharma Produktions GmbH
Active Ingredients
Documents
Package Inserts
Tabrecta FCT PI.pdf
Approved: November 6, 2022
