Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**2.2 Dosage and Method of Administration** **General** The safety and efficacy of alternating or switching between Pegasys and products that are biosimilar but not deemed interchangeable has not been established. Therefore, the benefit-risk of alternating or switching needs to be carefully considered. Treatment should be initiated only by a physician experienced in the treatment of patients with hepatitis B or C. Please refer to the full prescribing information of the medicinal products that are used in combination with Pegasys. When used in combination with ribavirin, please refer to the ribavirin prescribing information. **2.2.1 Standard Dosage** **_Chronic Hepatitis B_** The recommended dosage of Pegasys for both HBeAg-positive and HBeAg-negative chronic hepatitis B is 180 mcg once weekly by subcutaneous administration in the abdomen or thigh. The recommended duration of therapy is 48 weeks. **_Chronic Hepatitis C_**– treatment-naïve patients: The recommended dosage for Pegasys, alone or in combination with oral ribavirin, is 180 mcg once-weekly by subcutaneous administration in the abdomen or thigh. Ribavirin should be administered with food. The recommended duration of Pegasys monotherapy is 48 weeks. Please refer to the full prescribing information for ribavirin. The duration of combination therapy and the daily dose of ribavirin given in combination with Pegasys should be individualized based on the patient’s viral genotype (see Table 1). The use of Pegasys monotherapy in patients with normal ALT levels at baseline have not been studied in any clinical trials. 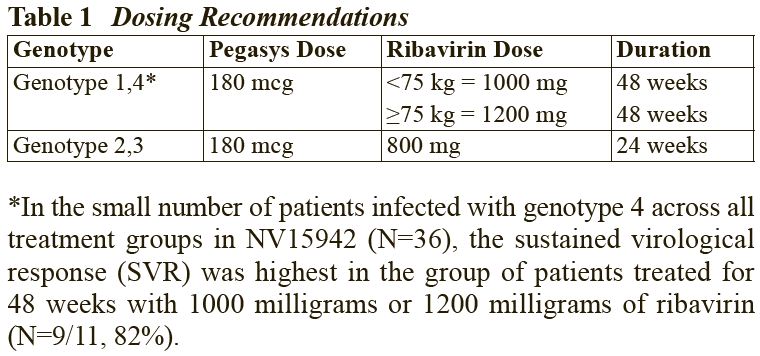 In general, patients infected with genotype 4 are considered hard to treat and limited study data (N=49) are compatible with a posology as for genotype 1. When deciding on the duration of therapy, the presence of additional risk factors should also be considered. For patients infected with genotype 5 or 6, this posology should also be considered. Chronic hepatitis C – treatment-experienced patients: The recommended dose of Pegasys in combination with ribavirin is 180 mcg once weekly by subcutaneous administration. For patients <75 kg and ≥75 kg, 1000 mg daily and 1200 mg daily of ribavirin, respectively, should be administered. Patients who have detectable virus at week 12 should stop therapy. The recommended duration of therapy is up to 72 weeks in genotype 1 or 4 patients and 48 weeks in genotype 2 or 3 patients. _HIV-HCV Co-infection_ The recommended dosage for Pegasys, alone or in combination with 800 milligrams of ribavirin, is 180 micrograms once weekly subcutaneously for 48 weeks, regardless of genotype. The safety and efficacy of combination therapy with ribavirin doses greater than 800 milligrams daily or a duration of therapy less than 48 weeks has not been studied. _Duration of therapy when Pegasys is used in combination with other medicinal products_ Please also refer to the full prescribing information of the medicinal products that are used in combination with Pegasys. _Predictability of response – Naïve patients_ Early virological response by week 12, defined as a 2 log viral load decrease or undetectable levels of HCV RNA has been shown to be predictive for sustained response (see Tables 2 and 8). 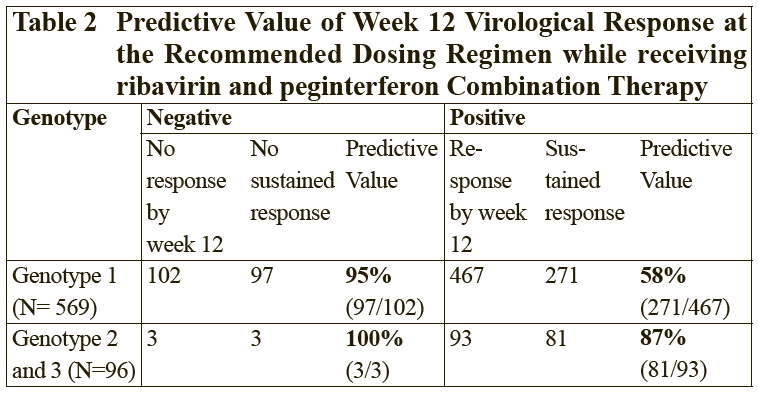 \[From 3.1.2 Efficacy / Clinical Studies\] 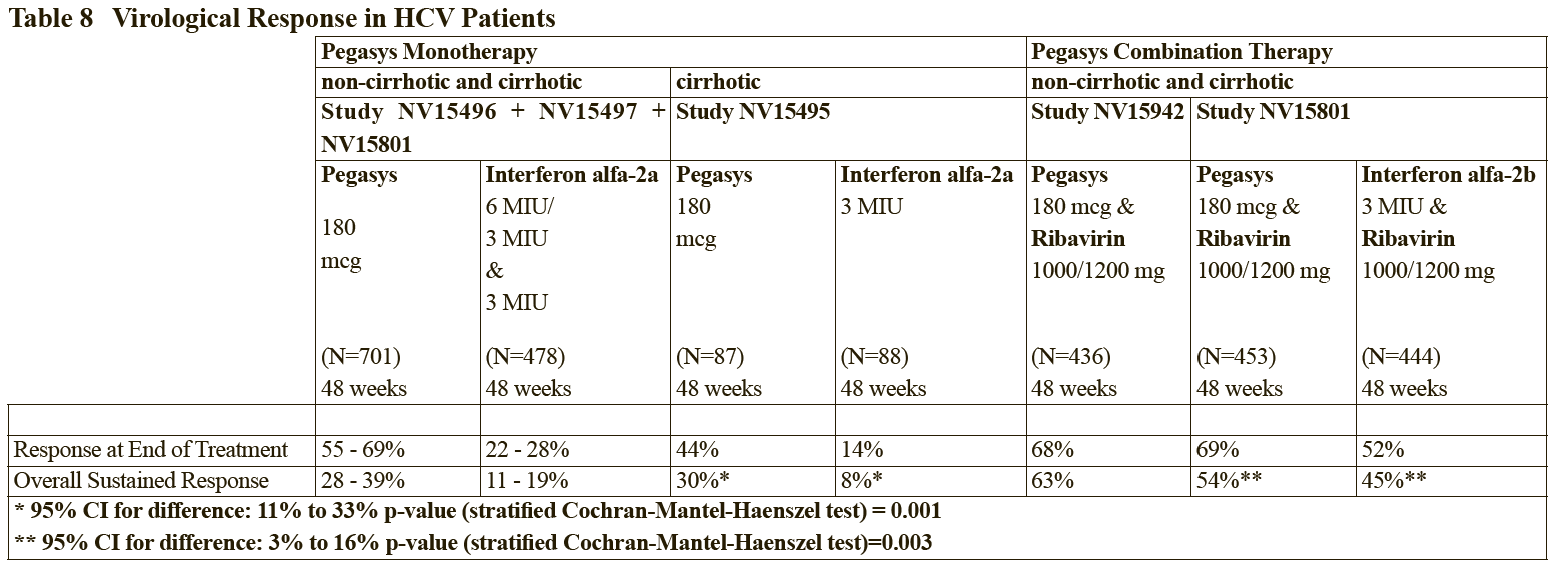 Patients treated with Pegasys who failed to achieve an early virological response were highly unlikely (<5%) to achieve a sustained virological response with continued therapy. In clinical studies, 97 of 102 difficult to treat patients (genotype 1) who received Pegasys and ribavirin combination therapy for 48 weeks and who failed to achieve an early virological response also failed to achieve a sustained virological response 24 weeks after therapy. This indicates a 95% negative predictability by week 12. Consideration should be given to discontinuing anti-viral therapy in these patients, especially if in a non-cirrhotic stage of disease. The negative predictive value of week 12 virological response for sustained response in patients treated with Pegasys in monotherapy was 98%. A similar negative predictive value has been observed in HIV-HCV co-infected patients treated with Pegasys monotherapy or in combination with ribavirin (100% (130/130) or 98% (83/85), respectively). Positive predictive values of 45% (50/110) and 70% (59/84) were observed, respectively, for genotype 1 and genotype 2/3 HIV-HCV co-infected patients receiving combination therapy. _Prior treatment-experienced patients_ In non-responder patients re-treated for 48 (genotype 2 and 3) or 72 weeks (genotype 1 and 4), viral suppression at week 12 (undetectable HCV RNA defined as <50 international units/mL) has shown to be predictive for sustained virological response. The probabilities of not achieving a sustained virological response with 48 or 72 weeks of treatment if viral suppression was not achieved at week 12 were 96% (363 of 380) and 96% (324 of 339), respectively. The probabilities of achieving a sustained virological response with 48 or 72 weeks of treatment if viral suppression was achieved at week 12 were 35% (20 of 57) and 57% (57 of 100), respectively. _Discontinuation of treatment_ Discontinuation of treatment is recommended if at least a 2 log10 reduction from baseline or undetectable HCV RNA has not been demonstrated by 12 weeks of therapy (see section Predictability of response – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Additionally, if patients have not achieved undetectable HCV RNA by week 24, therapy should be discontinued. **Dose modification** _General_ When dose modification is required for moderate to severe adverse reactions (clinical and/or laboratory), initial dose reduction to 135 mcg is generally adequate. However, in some cases, dose reduction to 90 mcg or 45 mcg is necessary. Dose increases to or toward the original dose may be considered when the adverse reaction abates (see sections 2.4 and 2.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hematological_ Dose reduction is recommended if the absolute neutrophil count (ANC) is less than 750 cells/mm3. For patients with ANC values below 500 cells/mm3, treatment should be suspended until ANC values return to more than 1000 cells/mm3. Therapy should initially be reinstituted at 90 mcg Pegasys and the neutrophil count monitored. Dose reduction to 90 mcg is recommended if the platelet count is less than 50,000 cells/mm3. Cessation of therapy is recommended if platelet count decreases to levels below 25,000 cells/mm3. _Dose modification for ribavirin in Chronic Hepatitis C when administered in combination therapy_ For management of treatment-emergent anemia, the dose of Ribavirin should be reduced to 600 mg per day (200 mg in the morning and 400 mg in the evening) if either of the following apply: - A patient without significant cardiovascular disease experiences a fall in hemoglobin levels to <10 g/dl and ≥8.5 g/dl or - A patient with stable cardiovascular disease experiences a fall in hemoglobin levels by ≥2 g/dl during any 4 weeks of treatment. Ribavirin should be _discontinued_ under the following circumstances: - If a patient without significant cardiovascular disease experiences a confirmed decrease in hemoglobin levels to <8.5 g/dl. - If a patient with stable cardiovascular disease maintains a hemoglobin value <12 g/dl despite 4 weeks on a reduced dose. Once the patient’s ribavirin dose has been withheld due to a laboratory abnormality or clinical manifestation an attempt may be made to restart ribavirin at 600 mg daily and further increase the dose to 800 mg daily depending upon the physician’s judgment. However, it is not recommended that ribavirin be increased to the original dose (1000 mg or 1200 mg). In case of intolerance to ribavirin, Pegasys monotherapy may be continued. 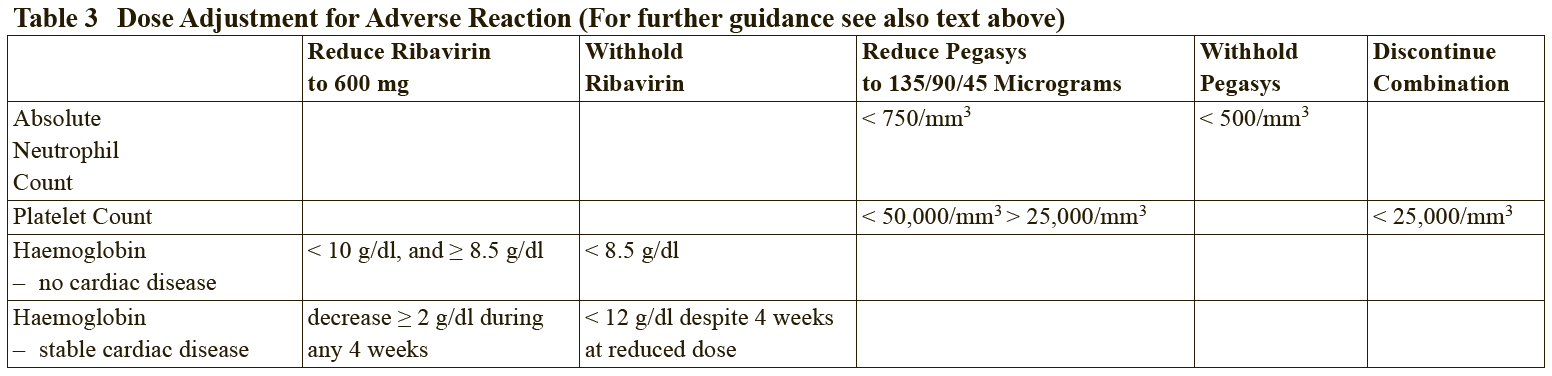 In case of intolerance to ribavirin, Pegasys monotherapy may be continued. **2.2.2 Special Dosage Instructions** **Geriatric use** No dose adjustment of Pegasys is required in patients ≥ 65 years of age. **Renal impairment** No dose adjustment is required for adult patients with mild or moderate renal impairment. A reduced dose of 135 mcg once weekly Pegasys is recommended in adult patients with severe renal impairment. In adult patients with end stage renal disease, a starting dose of Pegasys 135 mcg once weekly should be used (see section 3.2.5 Pharmacokinetic and Pharmacodynamics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Regardless of the starting dose or degree of renal impairment, patients should be monitored and appropriate dose reductions of Pegasys during the course of therapy should be made in the event of adverse reactions. No data is available for pediatric patients with renal impairment. **Hepatic impairment** Fluctuations in abnormalities of liver function tests are common in patients with chronic hepatitis. However, as with other alpha interferons, increases in ALT levels above baseline have been observed in patients treated with Pegasys, including patients with a virological response. For HCV patients, the dose should be reduced initially to 135 mcg in the presence of progressive or persistent ALT increases above baseline values. When increase in ALT levels is progressive despite dose reduction, or is accompanied by increased bilirubin or evidence of hepatic decompensation, therapy should be discontinued (see section Special Warnings and Special Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For HBV patients, transient flares of ALT levels sometimes exceeding 10 times the upper limit of normal are not uncommon, and may reflect immune clearance. Treatment should normally not be initiated if ALT ≥ 10 times the upper limit of normal. Consideration should be given to continuing treatment with more frequent monitoring of liver function during ALT flares. If the Pegasys dose is reduced or withheld, therapy can be restored once the flare is subsiding (see section Special Warnings and Special Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
SUBCUTANEOUS
Medical Information
**2.1 Therapeutic Indications** Pegasys is indicated in combination with other medicinal products, for the treatment of chronic hepatitis C (CHC) in patients with compensated liver disease. This includes patients with compensated cirrhosis and patients with HIV disease that is clinically stable (e.g. antiretroviral therapy not required or receiving stable antiretroviral therapy). The optimal way to use Pegasys in patients with chronic hepatitis C is in combination with ribavirin. The combination of Pegasys and ribavirin is indicated in naive patients and patients who have failed previous treatment with interferon alpha (pegylated or non-pegylated) alone or in combination therapy with ribavirin. Monotherapy is indicated mainly in case of intolerance or contraindication to ribavirin. Chronic Hepatitis B (CHB): Pegasys is indicated for the treatment of both HBeAg-positive and HBeAg-negative chronic hepatitis B in adult patients with compensated liver disease and evidence of viral replication and liver inflammation.
**2.3 Contraindications** Pegasys is contraindicated in: - patients with known hypersensitivity to alpha interferons, to _E. coli_-derived products, to polyethyleneglycol or to any component of the product. - patients with autoimmune hepatitis. - patients with severe hepatic dysfunction or decompensated cirrhosis. - neonates and infants up to 3 years of age. - A history of severe pre-existing cardiac disease, including unstable or uncontrolled cardiac disease in the previous six months (see section 2.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Initiation of Pegasys is contraindicated in HIV-HCV patients with cirrhosis and a Child-Pugh score ≥ 6 except if only due to indirect hyperbilirubinemia caused by drugs such as atazanavir and indinavir. - Pre-existing severe psychiatric condition or a history of severe psychiatric disorders, mainly depression. Due to the use of ribavirin, pregnant or breast feeding women must not be exposed to Pegasys/ribavirin combination therapy (please refer to section 2.5.1 Pregnancy and 2.5.2 Nursing Mothers – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L03AB11
peginterferon alfa-2a
Manufacturer Information
DKSH SINGAPORE PTE. LTD.
F HOFFMANN-LA ROCHE LTD
Active Ingredients
Documents
Package Inserts
Pegasys Pre-filled Syringe for Injection PI.pdf
Approved: October 10, 2022
