Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION
**4.2 Posology and method of administration** **Posology** The dose and dosage regimen is dependent on the indication. In replacement therapy, the dosage may need to be individualised for each patient dependent on the pharmacokinetic and clinical response. The following dosage regimens are given as a guideline. Replacement therapy in primary immunodeficiency syndromes The dosage regimen should achieve a trough level of IgG (measured before the next infusion) of at least 4–6 g/l. Three to six months are required after the initiation of therapy for equilibration to occur. The recommended starting dose is 0.4–0.8 g/kg followed by at least 0.2 g/kg every three weeks. The dose required to achieve a trough level of 6 g/l is of the order of 0.2–0.8 g/kg/month. The dosage interval when steady state has been reached varies from 2–4 weeks. Trough levels should be measured in order to adjust the dose and dosage interval. Replacement therapy in myeloma or chronic lymphocytic leukaemia with severe secondary hypogammaglobulinaemia and recurrent infections; replacement therapy in children with AIDS and recurrent infections The recommended dose is 0.2–0.4 g/kg every three to four weeks. Idiopathic Thrombocytopenic Purpura For the treatment of an acute episode, 0.8–1.0 g/kg on day one, which may be repeated once within 3 days, or 0.4 g/kg daily for two to five days. The treatment can be repeated if relapse occurs. Guillain Barré syndrome 0.4 g/kg/day for three to seven days. Experience in children is limited. Kawasaki disease 1.6–2.0 g/kg should be administered in divided dose over two to five days or 2.0 g/kg as a single dose. Patients should receive concomitant treatment with acetylsalicylic acid. Allogeneic bone marrow transplantation Human normal immunoglobulin treatment can be used as part of the conditioning regimen and after the transplant. For the treatment of infections and prophylaxis of graft versus host disease, dosage is individually tailored. The starting dose is normally 0.5 g/kg/week, starting seven days before transplantation and for up to three months after transplantation. In case of persistent lack of antibody production, dosage of 0.5 g/kg/month is recommended until antibody level returns to normal. The dosage recommendations are summarised in the following table: 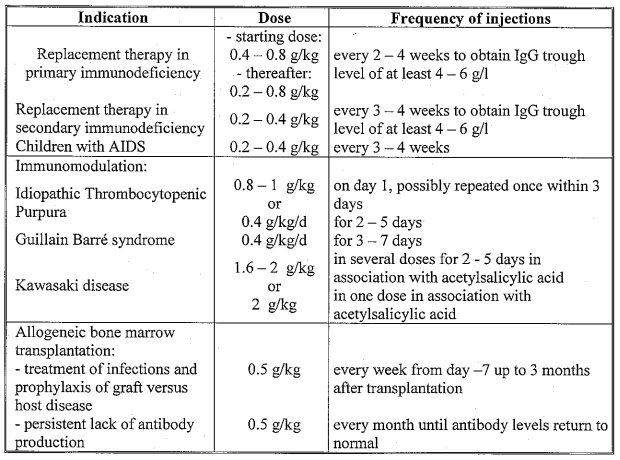 **Method of administration** Ig VENA should be infused intravenously at an initial rate of 0.46–0.92 ml/kg/hr (10–20 drops per minute) for 20–30 minutes. If well tolerated, the rate of administration may gradually be increased to a maximum of 1.85 ml/kg/hr (40 drops/minute) for the remainder of the infusion.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Replacement therapy in Primary immunodeficiency syndromes such as: - congenital agammaglobulinaemia and hypogammaglobulinaemia - common variable immunodeficiency - severe combined immunodeficiency - Wiskott Aldrich syndrome. Myeloma or chronic lymphocytic leukaemia with severe secondary hypogammaglobulinaemia and recurrent infections. Children with congenital AIDS and recurrent infections. Immunomodulation Idiopathic thrombocytopenic purpura (ITP), in children or adults at high risk of bleeding or prior to surgery to correct the platelet count. Guillain Barré syndrome. Kawasaki disease. Allogeneic bone marrow transplantation
**4.3 Contraindications** Hypersensitivity to any of the components. Hypersensitivity to homologous immunoglobulins, especially in very rare cases of IgA deficiency when the patient has antibodies against IgA.
J06BA02
immunoglobulins, normal human, for intravascular adm.
Manufacturer Information
DUO MEDICAL PTE. LTD.
Kedrion S.p.A.
Active Ingredients
Human normal immunoglobulin (Human plasma proteins containing at least 95% immunoglobulins)
50mg/ml
Documents
Package Inserts
Ig VENA PI.pdf
Approved: November 11, 2011
