Regulatory Information
GRIFOLS ASIA PACIFIC PTE. LTD.
GRIFOLS ASIA PACIFIC PTE. LTD.
Therapeutic
Prescription Only
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**DOSAGE AND ADMINISTRATION** Following reconstitution with the supplied diluent, Alphanate® should be administered intravenously within three hours after reconstitution to avoid the potential ill effect of any inadvertent bacterial contamination occurring during reconstitution. Alphanate® is administered by injection (plastic disposable syringes are recommended). Administer at room temperature, do not refrigerate after reconstitution, and discard any unused contents into the appropriate safety container. Antihemophilic Factor (AHF) potency (Factor VII:C activity) is expressed nominally in International Units (IU) on the product label. Additionally, each vial of Alphanate® also contains specific von Willebrand Factor:Ristocetin Cofactor (VWF:RCo) activity in international units for the treatment of VWD. **Hemophilia A** Dosing requirements and frequency of dosing is calculated on the basis of an expected initial response of 2% of normal FVIII:C increase per FVIII:C international units/kg body weight administered.21,22 The _in vivo_ increase in plasma Factor VIII can therefore be estimated by multiplying the dose of AHF per kilogram of body weight (FVIII:C international units/kg) by 2%. Thus, an administered AHF dose of 50 international units/kg will be expected to increase the circulating Factor VIII level by 100% of normal (100 international units/dL}. The following formulas and examples illustrate these principles:  Example: A 15 kg child with a baseline plasma FVIII level of 0%. To increase the plasma Factor VIII concentration to 100% of normal, the dosage required is as follows: 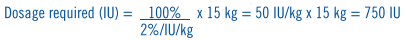 The following dosages are presented as general guidance as shown in **Table 3**. It should be emphasized that the dosage of Alphanate® required for hemostasis must be individualized according to the needs of the patient, the severity of the deficiency, the severity of the hemorrhage, the presence of inhibitors, and the FVIII level desired. Adequacy of treatment must be judged by the clinical effects and situation and thus, the dosage may vary with individual cases. 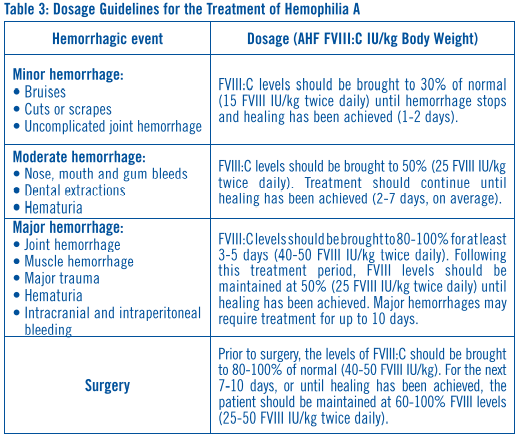 Dosing requirements and frequency of dosing is calculated on the basis of an expected initial response of 2% FVIII:C increase per FVIIIC international units/kg body weight (i.e., 2% per international units/kg) and an average half-life for FVIII:C of 12 hours.19,20 If dosing studies have determined that a particular patient exhibits a lower than expected response, the dose should be adjusted accordingly. Failure to achieve the expected plasma FVIII:C level or to control bleeding after an appropriately calculated dosage may be indicative of the development of an inhibitor (an antibody to FVIII:C). Its presence should be documented and the inhibitor level quantitated by appropriate laboratory procedures. Treatment with AHF in such cases must be individualized.15–17 Plasma factor VIII levels should be monitored periodically to evaluate individual patient response to the dosage regime. **Von Willebrand Disease** **Table 4** provides dosing guidelines for pediatric and adult patients with von Willebrand Disease.23–26 The amount of specific VWF:RCo and nominal Factor VIII contained in each vial of Alphanate® is indicated on the vial's label. The ratio of VWF:RCo to Factor VIII in Alphanate® varies by lot, so dosage should be re-evaluated whenever lot selection is changed. 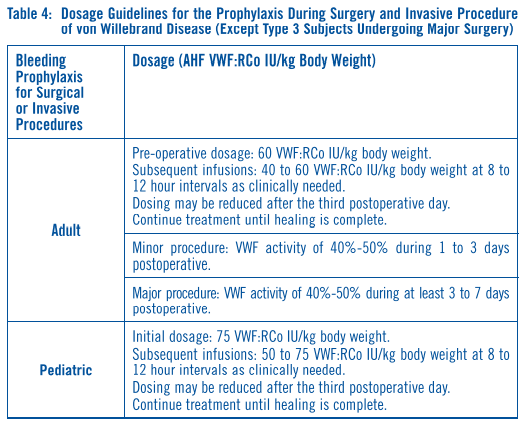 * * * 15\. Kasper, C.K. Incidence and Course of Inhibitors Among Patients with classic Hemophilia. Thromb Diath Haemorrh 1973; 30:263–271. 16\. Rizza, C.R., Biggs, R. The Treatment of Patients Who Have Factor VIII Antibodies Br J Haematol 1973; 24:65–82. 17\. Roberts, H.R., Knowles, M.R., Jones, T.L., McMillan, C. The Use of Factor VIII in the Management of Patients with Factor VIII Inhibitors. In: Brinkhous, K.M., ed. Hemophilia and New Hemorrhagic States, International Symposium, New York, University of North Carolina Press, 1970, pp. 152–163. 19\. Rizza, C.R., Biggs, R. Blood Products in the Management of Haemophilia and Christmas Disease. In: Poller, L., ed. Recent Advances in Blood Coagulation, Boston: Little Brown, 1969, pp. 179–195. 20\. Hathaway, W.E., Mahasandana, C., Clarke, S. Alteration of Platelet Function After Transfusion in Hemophilia. Proc 14th Ann Mtg, Am Soc Hematol 1971, Abstracts, 58, No. 88. 21\. Shanbrom, E., Thelin, M. Experimental Prophylaxis of Severe Hemophilia with a Factor VIII Concentrate. JAMA 1969; 208(9):1853–1856. 22\. Levine, P.H. Hemophilia and Allied Conditions. In: Brain, M.C. ed. Current Therapy in Hematology-Oncology: 1983–1984, New York: BC Decker, 1983, pp. 147–152. 23\. Federici, A.B., Baudo, F., Caracciolo, C., Mancuso, G., Mazzucconi, M.G., Musso, R., Schinco, P.C., Targhetta. R., Mannucci, P.M. Clinical efficacy of highly purified, doubly virus-inactivated factor VIII/von Willebrand factor concentrate (Fanhdi®) in the treatment of von Willebrand disease: a retrospective clinical study. Haemophilia 2002; 8:761–767. 24\. Federici, A.B. Management of von Willebrand disease with FVIII/von Willebrand factor concentrates: results from current studies and surveys. Blood Coagul Fibrinolysis 2005;16(Suppl 1):S17–S21. 25\. Mannucci, P.M. How I treat patients with von Willebrand disease. Blood 2001; 97:1915–1919. 26\. Mannucci, P.M. Treatment of von Willebrand's Disease. N Engl J Med 2004;351:683–694.
INTRAVENOUS
Medical Information
**INDICATIONS AND USE** **Hemophilia A or Acquired Factor VIII Deficiency** Antihemophilic Factor/von Willebrand Factor Complex (Human), Alphanate®, is indicated for the prevention and control of bleeding in patients with Factor VIll deficiency due to hemophilia A or acquired Factor VIIl deficiency.10 **Von Willebrand Disease** Antihemophilic Factor/von Willebrand Factor Complex (Human), Alphanate®, is also indicated for surgical and/or invasive procedure in patients with von Willebrand Disease (VWD), in whom desmopressin (DDAVP®) is either ineffective or contraindicated, except Type 3 patients undergoing major surgery. * * * 10\. Eyster, M.E. Hemophilia: A Guide for the Primary Care Physician. Postgrad Med 1978; 64:75–81
**CONTRAINDICATIONS** None known.
B02BD06
von Willebrand factor and coagulation factor VIII in combination
Manufacturer Information
GRIFOLS ASIA PACIFIC PTE. LTD.
GRIFOLS BIOLOGICALS LLC.
INSTITUTO GRIFOLS, S.A. (manufacturer for WFI)
ROVI PHARMA INDUSTRIAL SERVICES, S.A. (alternate manufacturer for WFI)
Grifols Therapeutics Inc. (Alternate Manufacturer for Cryoprecipitate)
Active Ingredients
Documents
Package Inserts
1.4.3 Revised Artwork Alphanate PI.pdf
Approved: March 14, 2019
