Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2 DOSAGE AND ADMINISTRATION** Due to the rapid reaction of the drug with hydrochloric acid in stomach, the dosage of Sevelamer Carbonate tablet is expected to be similar with hydrochloride. **2.1 General Dosing Information** The drug should be taken 3 times daily with meals. _Dose for Adult Patients Not Taking a Phosphate Binder:_ The recommended starting dose of Sevelamer Carbonate is 0.8 to 1.6 g with meals based on serum phosphorus level. Table 1 provides recommended starting doses of Sevelamer Carbonate for adult patients not taking a phosphate binder. 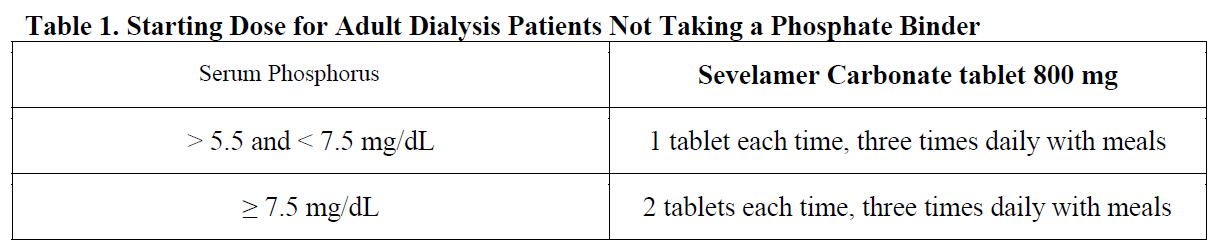 _Switching from Sevelamer Hydrochloride Tablets:_ For adult patients switching from Sevelamer Hydrochloride tablets to Sevelamer Carbonate tablets or powder, use the same dose in grams. To achieve the target phosphate concentration, further adjusting of dose may be necessary. For CKD patients on dialysis treatment, the highest daily dose of the used Sevelamer Carbonate in the study is 14 grams. _Switching from Calcium Acetate:_ A research performing on 84 of CKD patients on dialysis indicated that equivalent dose (approximate mg to mg) of Sevelamer Hydrochloride and Calcium Acetate showed similar decreasing serum phosphorus levels. Table 2 gives recommended starting doses of Sevelamer Carbonate based on a patient’s current calcium acetate dose. 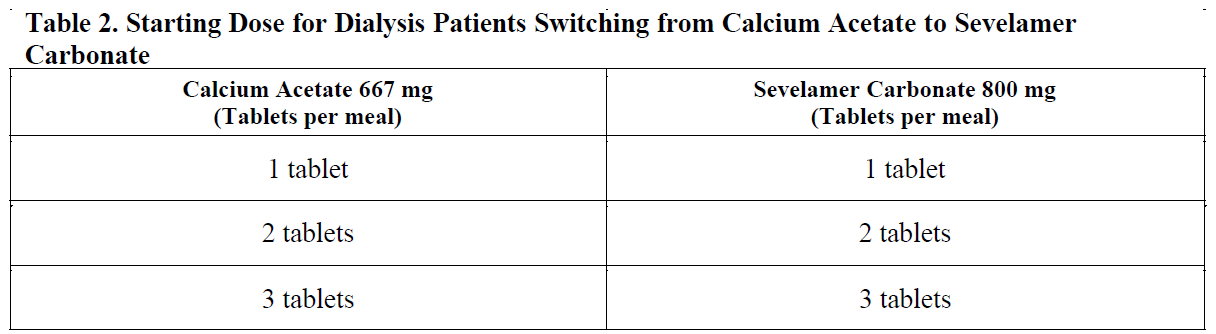 _Adjusting doses for all patients using this product:_ Sevelamer Carbonate dose by 0.8 g three times per day with meals at two-week intervals as necessary to achieve target serum phosphorus levels (3.5 mg/dL to 5.5 mg/dL). Sevelamer Carbonate tablet should be swallowed entirely and should not be grinded, chewed to fragments or split.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** Sevelamer Carbonate is indicated for the control of hyperphosphatemia in adult patients receiving haemodialysis or peritoneal dialysis. Sevelamer Carbonate should be used within the context of a multiple therapeutic approach, which could include calcium supplement, 1,25-dihydroxyvitamin D3 or one of its analogues to control the development of renal bone disease.
**4 CONTRAINDICATIONS** Sevelamer carbonate is contraindicated in patients with hypophosphatemia and bowel obstruction. Sevelamer carbonate is contraindicated in patients with known hypersensitivity to sevelamer carbonate, or to any of the excipients.
V03AE02
sevelamer
Manufacturer Information
MD PHARMACEUTICALS PTE. LTD.
Chen Ho Pharmaceutical Co., Ltd. Sinying Plant
Active Ingredients
Documents
Package Inserts
Sevela film coated tablet 800mg PI.pdf
Approved: February 16, 2021
