Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and method of administration** Treatment with CALQUENCE should be initiated and supervised by a physician experienced in the use of anticancer therapies. **Posology** _**MCL**_ The recommended dose of CALQUENCE for the treatment of MCL is 100 mg (1 capsule) twice daily. _**CLL/SLL**_ The recommended dose of CALQUENCE for the treatment of CLL/SLL is 100 mg (1 capsule) twice daily, either as monotherapy or in combination with obinutuzumab. Refer to the obinutuzumab prescribing information for recommended obinutuzumab dosing information. (For details of the combination regimen, see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Doses should be separated by approximately 12 hours. Treatment with CALQUENCE should continue until disease progression or unacceptable toxicity. **Missed Dose** If a patient misses a dose of CALQUENCE by more than 3 hours, instruct the patient to take the next dose at its regularly scheduled time. Extra capsules of CALQUENCE should not be taken to make up for a missed dose. **Dose Adjustments** **_Adverse Reactions_** Recommended dose modifications of CALQUENCE for Grade ≥ 3 adverse reactions are provided in Table 1. Temporarily interrupt CALQUENCE to manage a Grade ≥ 3 non-haematological treatment-related adverse reaction, Grade 3 thrombocytopenia with significant bleeding, Grade 4 thrombocytopenia, or Grade 4 neutropenia lasting longer than 7 days. Upon resolution of the adverse reaction to Grade 1 or baseline (recovery), restart CALQUENCE as recommended in Table 1. 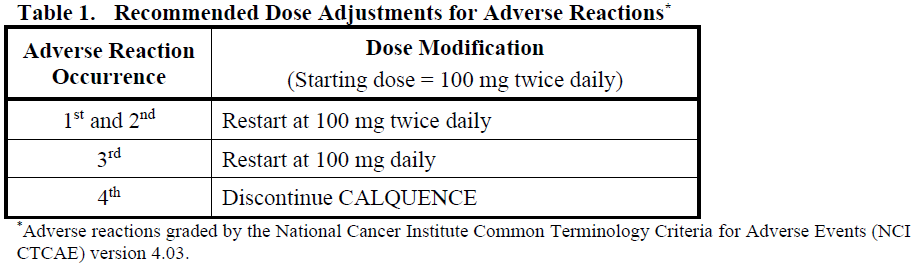  **Method of Administration** CALQUENCE should be swallowed whole with water at approximately the same time each day. CALQUENCE can be taken with or without food. The capsule should not be chewed, dissolved, or opened. **Special patient populations** **_Elderly (≥ 65 years)_** No dose adjustment is necessary based on age (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **_Renal Impairment_** No dose adjustment is recommended in patients with mild to moderate renal impairment (eGFR greater than or equal to 30 mL/min/1.73m2 as estimated by MDRD (modification of diet in renal disease equation)). The pharmacokinetics and safety of CALQUENCE in patients with severe renal impairment (eGFR less than 29 mL/min/1.73m2) or end-stage renal disease have not been studied (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **_Hepatic Impairment_** No dose adjustment is recommended in patients with mild or moderate hepatic impairment (Child-Pugh A, Child-Pugh B, or total bilirubin between 1.5–3 times the upper limit of normal \[ULN\] and any AST). Avoid administration of CALQUENCE in patients with severe hepatic impairment (Child-Pugh C or total bilirubin > 3 times ULN and any AST) (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **_Severe Cardiac Disease_** Patients with severe cardiovascular disease were excluded from CALQUENCE clinical studies. **_Paediatric and adolescents_** The safety and efficacy of CALQUENCE in children and adolescents aged less than 18 years have not been established.
ORAL
Medical Information
**4.1 Therapeutic indications** CALQUENCE (acalabrutinib) is indicated: - in combination with obinutuzumab or as monotherapy for the treatment of patients with previously untreated chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). - as monotherapy for the treatment of patients with CLL/SLL who have received at least one prior therapy. - for the treatment of patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
**4.3 Contraindications** Hypersensitivity to acalabrutinib or to any of the excipients in the formulation.
pending
xpending
Manufacturer Information
ASTRAZENECA SINGAPORE PTE LTD
AstraZeneca AB
Active Ingredients
Documents
Package Inserts
Calquence Capsule PI.pdf
Approved: May 11, 2023
