Regulatory Information
PFIZER PRIVATE LIMITED
PFIZER PRIVATE LIMITED
Therapeutic
Prescription Only
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** **Dosage** Treatment should be initiated under the supervision of a physician experienced in the treatment of hemophilia. Treatment with all factor IX products, including BeneFIX, requires individualized dosage adjustment. The dosage and duration of treatment for all factor IX products depends on the severity of the factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition. Dosing of BeneFIX may differ from that of plasma-derived factor IX products. To ensure that the desired factor IX activity level has been achieved, precise monitoring using the factor IX activity assay is advised, in particular for surgical interventions. In order to adjust the dose as appropriate, doses should be titrated taking into consideration factor IX activity, pharmacokinetic parameters, such as half-life and recovery, as well as the clinical situation. The number of units of factor IX administered is expressed in International Units (IU), which are related to the current WHO standard for factor IX products. Factor IX activity in plasma is expressed either as a percentage (relative to normal human plasma) or in International Units (relative to an international standard for factor IX in plasma). One International Unit (IU) of factor IX activity is equivalent to that quantity of factor IX in one ml of normal human plasma. Estimation of the required dose of BeneFIX can be based on the finding that one unit of factor IX activity per kg body weight is expected to increase the circulating level of factor IX, an average of 0.8 international units/dL (range from 0.4 to 1.4 international units/dL) in adult patients (≥15 years). Pharmacokinetics have to be assessed regularly in each patient and posology has to be adjusted accordingly. 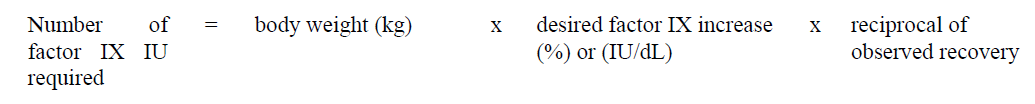 Use in Adults In adult PTPs, on average, one international unit of BeneFIX per kilogram of body weight increased the circulating activity of factor IX by 0.8 ± 0.2 (range 0.4 to 1.4) international units/dL. The method of dose estimation is illustrated in the following example. If you use 0.8 international units/dL average increase of factor IX per international units/kg body weight administered, then: 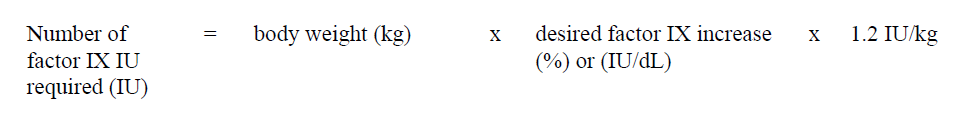 Dosage for Bleeding Episodes and Surgery In the case of the hemorrhagic events listed in the table below, the factor IX activity should not fall below the given plasma activity levels (in % of normal or in international units/dL) in the corresponding period. 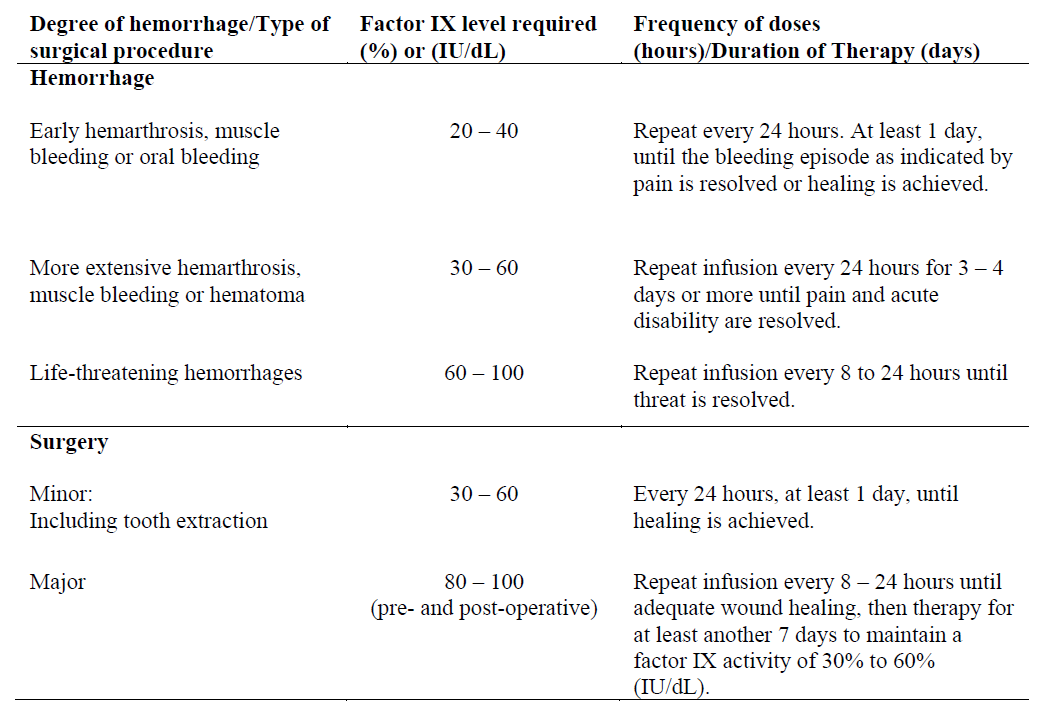 Dosage for Prophylaxis For long-term prophylaxis against bleeding in patients with severe hemophilia B, BeneFIX may be administered. In a clinical study for routine secondary prophylaxis the average dose for previously treated adult patients (PTP) was 40 international units/kg (range 13 to 78 international units/kg) at intervals of 3 to 4 days. In younger patients, shorter dosage intervals or higher doses may be necessary. **Once-weekly 100 international units/kg Dosing Regimen** In PTPs with hemophilia B (FIX:C≤2%), BeneFIX had been administered as 100 international units/kg once weekly in clinical trials. There was limited data to demonstrate that the factor IX activity level could be maintained at >1% throughout the dosing interval (see section 5.1 **Pharmacodynamic properties** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Close monitoring of the trough concentrations and/or presence of breakthrough bleeds should be undertaken and the dosing regimen (dose or frequency) should be adjusted accordingly. **Use in Pediatric** There are insufficient data to recommend the use of BeneFIX in children less than 6 years of age. In clinical studies, 57% of the pediatric patients increased their doses due to lower than expected recovery or to obtain sufficient therapeutic response or both, some to an average dose of >50 international units/kg. Therefore, close monitoring of factor IX plasma activity should be performed, as well as calculation of pharmacokinetic parameters, such as recovery and half-life, as clinically indicated, in order to adjust doses as appropriate. If doses >100 international units/kg have been repeatedly needed during routine prophylaxis or treatment, a switch to another FIX product should be considered. Patients should be monitored for the development of factor IX inhibitors. If the expected factor IX activity plasma levels are not attained, or if bleeding is not controlled with an appropriate dose, biological testing should be performed to determine if a factor IX inhibitor is present. In patients with high levels of inhibitor factor IX therapy may not be effective and other therapeutic options must be considered. Management of such patients should be directed by physicians with experience in the care of patients with hemophilia. See also section 4.4 **Special warnings and precautions for use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **Use in Elderly** Clinical studies of BeneFIX did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. As with any patient receiving BeneFIX, dose selection for an elderly patient should be individualized. **Method of Administration** BeneFIX is administered intravenously after reconstitution of the lyophilized powder for solution for injection with the supplied diluent (see section 6.6 **Special precautions for disposal and other handling** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). It should be injected over several minutes. The rate of administration should be determined by the patient's comfort level. Reconstituted BeneFIX should not be administered in the same tubing or container with other medicinal products. Following completion of BeneFIX treatment, remove the infusion set and discard. Dispose of all unused solution, empty vial(s), and used needles and syringes in an appropriate container for throwing away waste that might hurt others if not handled properly. The safety and efficacy of administration by continuous infusion have not been established. See also section 4.4 **Special warnings and precautions for use** and section 4.8 **Undesirable effects** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. BeneFIX, when reconstituted, contains polysorbate-80, which is known to increase the rate of di-(2-ethylhexyl) phthalate (DEHP) extraction from polyvinyl chloride (PVC). This should be considered during the preparation and administration of BeneFIX, including storage time elapsed in a PVC container following reconstitution. It is important that the recommendations in section 4.2 **Posology and method of administration** be followed closely. BeneFIX should be administered using the infusion set provided in the kit, and the pre-filled diluent syringe provided or a single, sterile, disposable plastic syringe. In addition, the solution should be withdrawn from the vial using the vial adapter. The reconstituted solution may be stored at room temperature prior to administration. However, BeneFIX should be administered within 3 hours after reconstitution. Note: Agglutination of red blood cells in the tubing/syringe has been reported with the administration of BeneFIX. No adverse events have been reported in association with this observation. To minimize the possibility of agglutination, it is important to limit the amount of blood entering the tubing. Blood should not enter the syringe. If red blood cell agglutination is observed in the tubing or syringe, discard all material (tubing, syringe and BeneFIX solution) and resume administration with a new package. **Reconstitution** Always wash your hands before performing the following procedures. Aseptic technique (meaning clean and germ-free) should be used during the reconstitution procedure. All components used in the reconstitution and administration of this product should be used as soon as possible after opening their sterile containers to minimize unnecessary exposure to the atmosphere. BeneFIX is administered by IV infusion after reconstitution with the supplied diluent (0.234% sodium chloride diluent) in the pre-filled syringe. 01. Allow the vial of lyophilized BeneFIX and the pre-filled diluent syringe to reach room temperature. 02. Remove the plastic flip-top cap from the BeneFIX vial to expose the central portions of the rubber stopper.  03. Wipe the top of the vial with the alcohol swab provided, or use another antiseptic solution, and allow to dry. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface. 04. Peel back the cover from the clear plastic vial adapter package. Do not remove the adapter from the package. 05. Place the vial on a flat surface. While holding the adapter in the package, place the vial adapter over the vial. Press down firmly on the package until the adapter snaps into place on the top of the vial, with the adapter spike penetrating the vial stopper. Leave the adapter package in place. 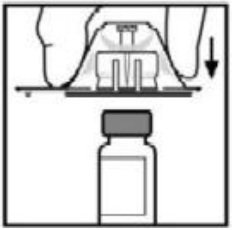 06. Grasp the plunger rod as shown in the diagram. Avoid contact with the shaft of the plunger rod. Attach the threaded end of the plunger rod to the diluent syringe plunger by pushing and turning firmly. 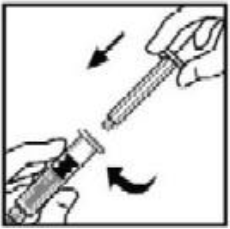 07. Remove the tamper-resistant, plastic-tip cap from the diluent syringe by bending the cap up and down to break the perforation. Do not touch the inside of the cap or the syringe tip. Place the cap on its side on a clean surface in a spot where it would be least likely to become environmentally contaminated. 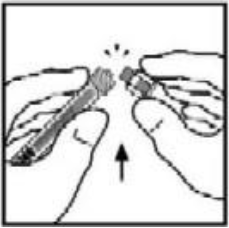 08. Lift the package away from the adapter and discard the package. 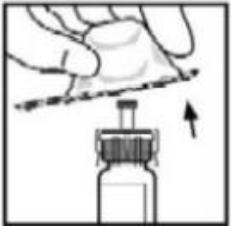 09. Place the vial on a flat surface. Connect the diluent syringe to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until the connection is secured. 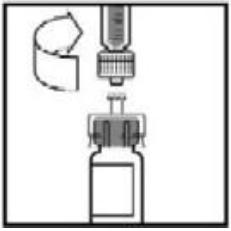 10. Slowly depress the plunger rod to inject all the diluent into the BeneFIX vial. 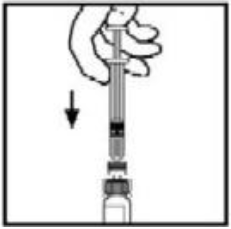 11. Without removing the syringe, gently swirl the contents of the vial until the powder is dissolved. 12. Inspect the final solution for specks before administration. The solution should appear clear and colorless. Note: If you use more than one vial of BeneFIX per infusion, reconstitute each vial by following the previous instructions. 13. Ensuring that the syringe plunger rod is still fully depressed, invert the vial. Slowly draw the solution into the syringe. 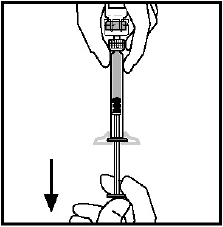 Note: If you prepared more than one vial of BeneFIX, remove the diluent syringe from the vial adapter, leaving the vial adapter attached to the vial. Quickly attach a separate large luer lock syringe and draw back the reconstituted contents as instructed above. Repeat this procedure with each vial in turn. Do not detach the diluent syringes or the large luer lock syringe until you are ready to attach the large luer lock syringe to the next vial adapter. 14. Detach the syringe from the vial adapter by gently pulling and turning the syringe counterclockwise. Discard the vial with the adapter attached. Note: If the solution is not to be used immediately, the syringe cap should be carefully replaced. Do not touch the syringe tip or the inside of the cap. BeneFIX should be administered within 3 hours after reconstitution. The reconstituted solution may be stored at room temperature prior to administration.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indication** Treatment and prophylaxis of bleeding in patients with hemophilia B (congenital factor IX deficiency).
**4.3 Contraindications** BeneFIX may be contraindicated in patients with a known history of hypersensitivity to any of the constituents of the preparation or in patients with a known history of hypersensitivity to hamster proteins.
B02BD04
coagulation factor IX
Manufacturer Information
PFIZER PRIVATE LIMITED
(Diluent) Vetter Pharma-Fertigung GmbH & Co. KG
Wyeth Farma S.A
Vetter Pharma-Fertigung GmbH & Co. KG (Manufacturer of diluent)
Active Ingredients
Documents
Package Inserts
BeneFIX Powder and Solvent for Solution for Injection PI.pdf
Approved: October 22, 2021
