Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE, GELATIN COATED
**2 DOSAGE AND ADMINISTRATION** Administration One AKYNZEO capsule administered approximately 1 hour prior to the start of chemotherapy. Highly Emetogenic Chemotherapy, including Cisplatin Based Chemotherapy The recommended dosage in adults is one capsule of AKYNZEO administered approximately 1 hour prior to the start of chemotherapy with dexamethasone 12 mg administered orally 30 minutes prior to chemotherapy on day 1 and 8 mg orally once daily on days 2 to 4 _\[see Clinical Studies (14), Table 5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. \[From **14 CLINICAL STUDIES**\] 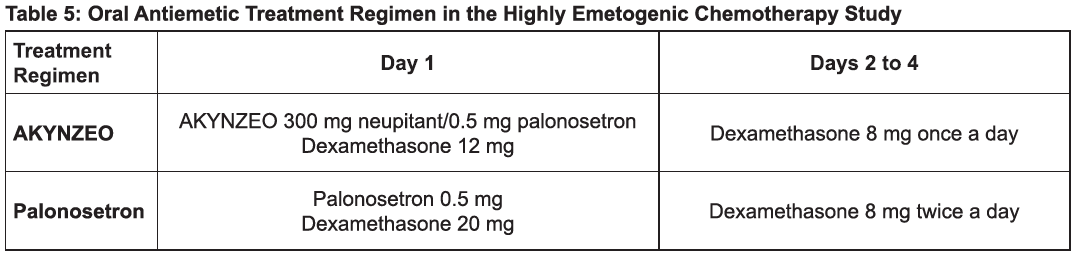 Anthracyclines and Cyclophosphamide Based Chemotherapy and Chemotherapy Not Considered Highly Emetogenic The recommended dosage in adults is one capsule of AKYNZEO approximately 1 hour prior to the start of chemotherapy with dexamethasone 12 mg administered orally 30 minutes prior to chemotherapy on day 1. Administration of dexamethasone on days 2 to 4 is not necessary _\[see Clinical Studies (14), Table 7_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. \[From **14 CLINICAL STUDIES**\] 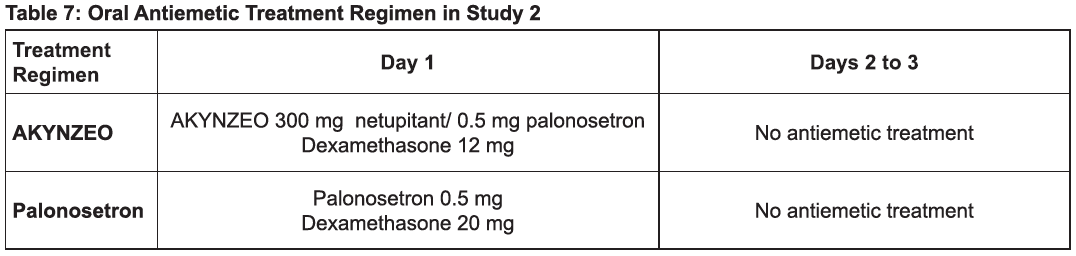 AKYNZEO can be taken with or without food.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** AKYNZEO is indicated for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy.
**4 CONTRAINDICATIONS** None.
A04AA55
palonosetron, combinations
Manufacturer Information
juniper healthcare pte ltd
Helsinn Birex Pharmaceuticals Ltd. (Intermediate Netupitant Tablets and final drug product)
CATALENT PHARMA SOLUTIONS LLC (Intermediate Palonosetron softgels)
Active Ingredients
Documents
Package Inserts
Akynzeo Capsules PI.pdf
Approved: March 17, 2022
