Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**Posology and method of administration** The average activity administered by single intravenous injection is 500 MBq (300 – 700 MBq) in a 50 to 70 kg adult. Other activities may be justifiable. There is no special dosage regimen for the elderly patient. The dose to be administered to a child should be a fraction of the adult dose calculated from the body weight according to the following table. 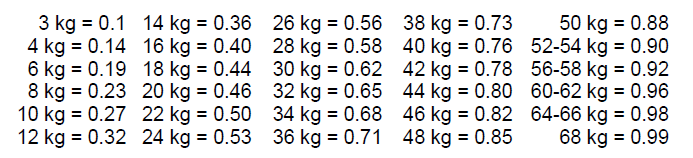 In very young children (up to 1 year) a minimum dose of 40 MBq is necessary in order to obtain images of sufficient quality. Images obtained shortly after injection (e.g. in the so-called "3-phase bone scan" procedure) will only partly reflect metabolic activity. Late phase static scintigraphy should be performed not earlier than 2 hours after injection. The patient should void before scanning.
INTRAVENOUS
Medical Information
**Therapeutic indications** After reconstitution with Sodium Pertechnetate (99mTc) Injection (Fission or Non-Fission) the agent may be used for bone scintigraphy, where it delineates areas of altered osteogenesis.
**Contra-indications** There are no specific contra-indications.
V09BA01
technetium (99mTc) oxidronic acid
Manufacturer Information
QT INSTRUMENTS (S) PTE LTD
Curium Netherlands B.V.
Active Ingredients
Documents
Package Inserts
Technescan HDP PI.pdf
Approved: January 11, 2022
