Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SUSPENSION
**Dosage and Administration** **Pharmaceutical Form** Film-coated tablet and suspension for injection **Posology** Therapy should be initiated by a physician experienced in the management of HIV infection. _VOCABRIA_ is indicated for the treatment of HIV in combination with rilpivirine, therefore, the prescribing information for rilpivirine should be consulted for recommended dosing. Prior to starting _VOCABRIA_, healthcare professionals should have carefully selected patients who agree to the required injection schedule and counsel patients about the importance of adherence to scheduled dosing visits to help maintain viral suppression and reduce the risk of viral rebound and potential development of resistance with missed doses. **Method of Administration** **Film-coated Tablet** _VOCABRIA_ may be taken with or without food. When taken at the same time as rilpivirine, _VOCABRIA_ should be taken with a meal. **Suspension for Injection** Refer to the Instructions for Use for detailed step by step injection procedure ( _see Instructions for Use & Handling_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _VOCABRIA_ injection should be administered by a healthcare professional. When administering the _VOCABRIA_ injection, healthcare professionals should take into consideration the BMI of the patient to ensure that the needle length is sufficient to reach the gluteus muscle. Cabotegravir and rilpivirine injections should be administered at separate gluteal injection sites during the same visit. **Adults** **_Oral lead-in_ (Film-coated Tablets)** When used for oral lead-in _VOCABRIA_ oral tablets are recommended for approximately one month (at least 28 days) in virologically suppressed patients prior to the initiation of _VOCABRIA_ injection to assess tolerability to cabotegravir. _VOCABRIA_ tablets should be taken together with rilpivirine tablets. **Monthly Dosing (Suspension for Injection)** _**Initiation Injection**_ On the final day of oral lead-in, the recommended initial _VOCABRIA_ injection dose in adults is a single 3 mL (600 mg) intramuscular injection. _**Continuation Injection**_ After the initiation injection, the recommended _VOCABRIA_ continuation injection dose in adults is a single 2 mL (400 mg) intramuscular injection, administered monthly. Patients may be given injections up to 7 days before or after the date of the monthly 2 mL dosing schedule. 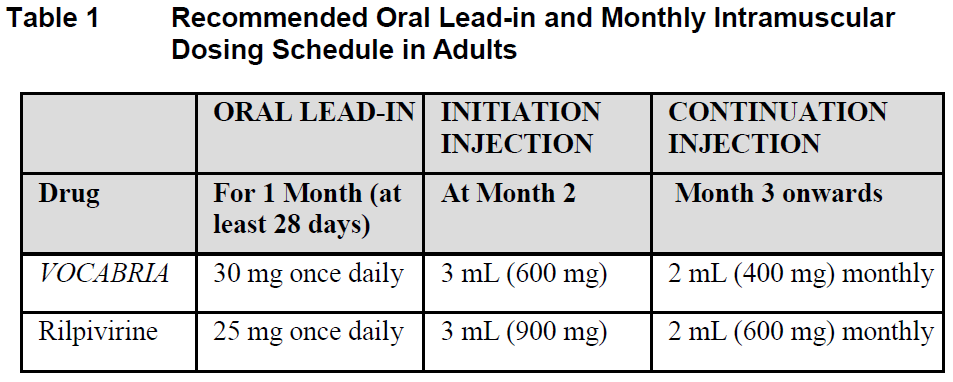 **Every 2 Month Dosing (Suspension for Injection)** **_Initiation Injections_** On the final day of oral lead-in, the recommended initial _VOCABRIA_ injection dose in adults is a single 3 mL (600 mg) intramuscular injection. One month later, a second 3 mL (600 mg) intramuscular injection should be administered. Patients may be given the second 3 mL (600 mg) initiation injection up to 7 days before or after the scheduled dosing date. _**Continuation Injections**_ After the second initiation injection, the recommended _VOCABRIA_ continuation injection dose in adults is a single 3 mL (600 mg) intramuscular injection administered every 2 months. Patients may be given injections up to 7 days before or after the date of the every 2 month, 3 mL dosing schedule. 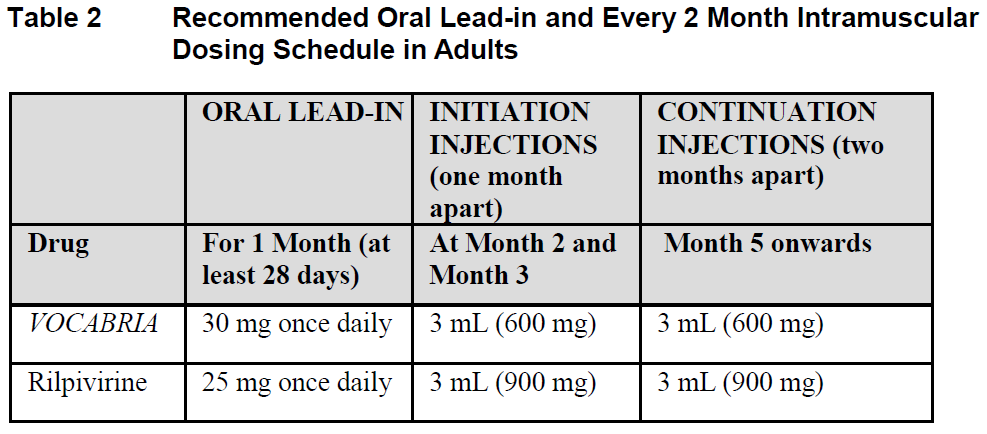 **Change in Dosing Frequency** **Dosing Recommendations when Switching from Monthly to Every 2 Month Injections** Patients switching from a monthly continuation injection schedule to an every 2 month continuation injection dosing schedule should receive a single 3 mL (600 mg) intramuscular injection of _VOCABRIA_ one month after the last 2 mL (400 mg) continuation injection dose and then 3 mL (600 mg) every 2 months thereafter. **Dosing Recommendations when Switching from Every 2 Month to Monthly Injections** Patients switching from an every 2 month continuation injection schedule to a monthly continuation dosing schedule should receive a single 400 mg intramuscular injection of _VOCABRIA_ 2 months after the last 600 mg continuation injection dose and then 400 mg monthly thereafter. _**Missed dose**_ **Film-coated Tablet** If the patient misses a dose of oral _VOCABRIA_, the patient should take the missed dose as soon as possible. **Suspension for Injection** Adherence to the injection dosing schedule is strongly recommended. Patients who miss a scheduled injection visit should be clinically reassessed to ensure resumption of therapy remains appropriate (see Tables 3 and 4). _Missed monthly injection_ If a delay of more than 7 days from a scheduled injection visit cannot be avoided, _VOCABRIA_ tablets (30 mg) may be used in combination with rilpivirine tablets (25 mg) once daily to replace up to 2 consecutive monthly injection visits. For oral therapy durations greater than two months, an alternative oral regimen is recommended. The first dose of oral therapy should be taken one month (+/–7 days) after the last injection dose of _VOCABRIA_ or rilpivirine. Injection dosing should be resumed on the day oral dosing completes, as recommended in Table 3. 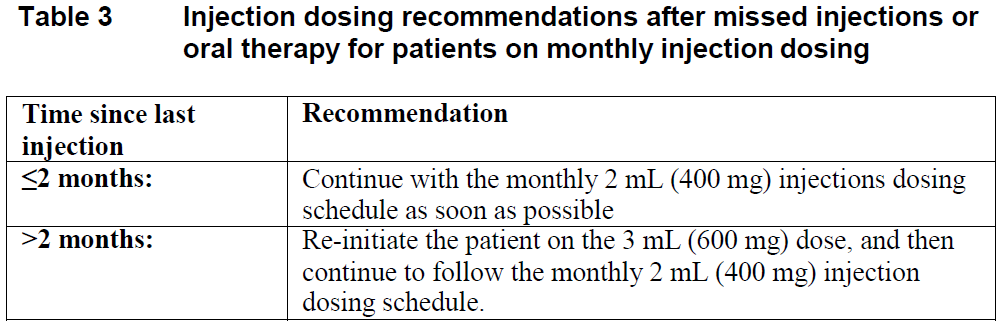 _Missed 2 month injection_ If a delay of more than 7 days from a scheduled injection visit cannot be avoided, _VOCABRIA_ tablets (30 mg) may be used in combination with rilpivirine tablets (25 mg) once daily to replace one 2-monthly injection visit. For oral therapy durations greater than two months, an alternative oral regimen is recommended. The first dose of oral therapy should be taken two months (+/–7 days) after the last injection dose of _VOCABRIA_ or rilpivirine. Injection dosing should be resumed on the day oral dosing completes, as recommended in Table 4. 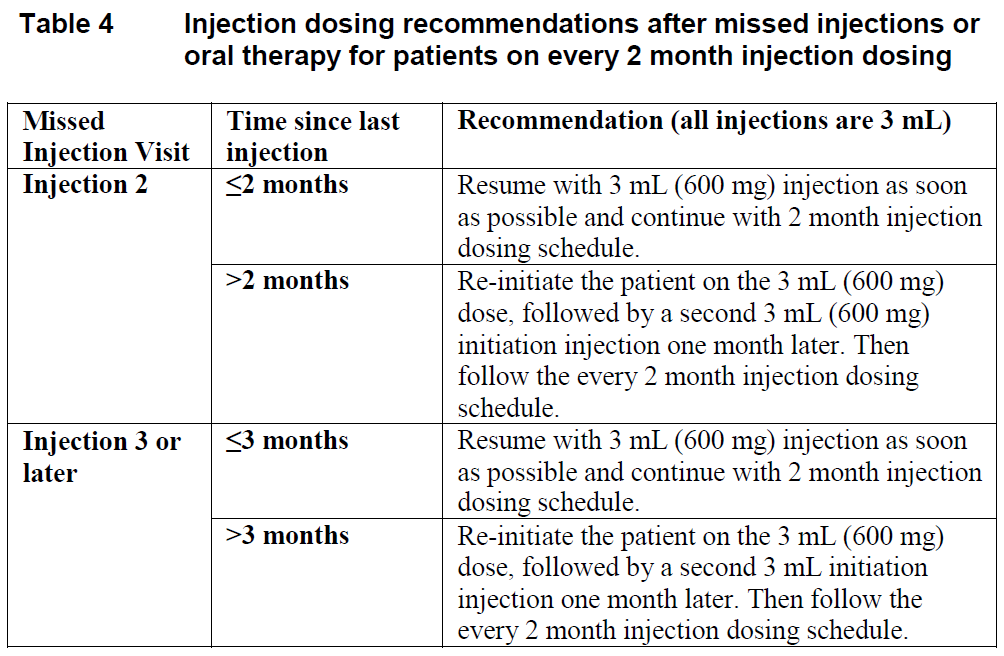 **Adolescents and Children** The safety and efficacy of _VOCABRIA_ in children and adolescents aged under 18 years has not been established. **Elderly** No dose adjustment is required in elderly patients. There are limited data available on the use of _VOCABRIA_ in patients aged 65 years and over ( _see Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal impairment** No dosage adjustment is required in patients with mild to severe renal impairment and not on dialysis ( _see Pharmacokinetics - Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Cabotegravir has not been studied in patients with end-stage renal disease on renal replacement therapy. **Hepatic impairment** No dosage adjustment is required in patients with mild or moderate hepatic impairment (Child-Pugh score A or B). _VOCABRIA_ has not been studied in patients with severe hepatic impairment (Child-Pugh score C) ( _see Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAMUSCULAR
Medical Information
**Indications** **Film-coated Tablets:** _VOCABRIA_ tablets are indicated in combination with rilpivirine tablets for short term ( _see Dosage and Administration_) treatment of human immunodeficiency virus (HIV)-1 infection in adults who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with agents of the NNRTI and INI class: - oral lead-in to assess tolerability of cabotegravir prior to administration of long acting (LA) _VOCABRIA_ injection. - oral therapy for adults who will miss planned dosing with _VOCABRIA_ injection. **Suspension for Injection:** _VOCABRIA_ injection is indicated in combination with rilpivirine injection for treatment of HIV-1 infection in adults who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with agents of the NNRTI and INI class ( _see Clinical studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**Contraindications** _VOCABRIA_ is contraindicated in patients: - with known hypersensitivity to cabotegravir or to any of the excipients in the tablets or the injection formulation. - receiving rifampicin, rifapentine, phenytoin, phenobarbital, carbamazepine and oxcarbazepine. _VOCABRIA_ is only indicated for treatment of HIV in combination with rilpivirine, therefore, the prescribing information for rilpivirine should also be consulted.
J05AJ04
cabotegravir
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
Glaxo Operations UK Limited (trading as Glaxo Wellcome Operations)
Sterigenics Belgium (Fleurus) (Sterilization)
Active Ingredients
Documents
Package Inserts
Vocabria PI.pdf
Approved: July 5, 2023
