Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SUSPENSION, EXTENDED RELEASE
**4.2 Posology and method of administration** For patients who have never taken aripiprazole, tolerability with oral aripiprazole must occur prior to initiating treatment with Abilify Maintena. Titration of the dose for Abilify Maintena is not required. The starting dose can be administered by following one of two regimens: - One injection start: On the day of initiation, administer one injection of 400 mg Abilify Maintena and continue treatment with 10 mg to 20 mg oral aripiprazole per day for 14 consecutive days to maintain therapeutic aripiprazole concentrations during initiation of therapy. - Two injection start: On the day of initiation, administer two separate injections of 400 mg Abilify Maintena at separate injection sites (see method of administration), along with one 20 mg dose of oral aripiprazole. After the injection start, the recommended maintenance dose of Abilify Maintena is 400 mg. Abilify Maintena should be administered once monthly as a single injection (no sooner than 26 days after the previous injection). If there are adverse reactions with the 400 mg dosage, reduction of the dose to 300 mg once monthly should be considered. Missed doses 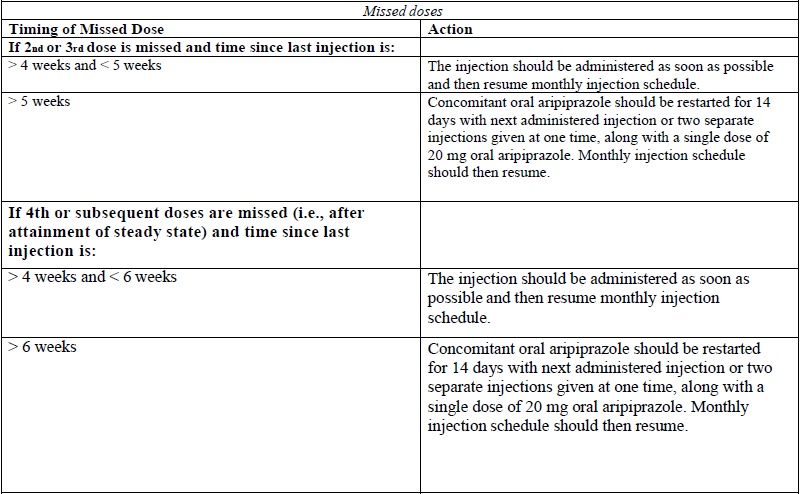 Special populations _Elderly patients_ The effectiveness and safety of Abilify Maintena in the treatment of schizophrenia in patients > 61 years and in the treatment of bipolar I disorder in patients ≥ 66 years have not been evaluated. _Renal impairment_ No dosage adjustment is required for patients with renal impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ No dosage adjustment is required for patients with mild or moderate hepatic impairment. In patients with severe hepatic impairment, the data available are insufficient to establish recommendations. In these patients requiring cautious dosing, oral formulation should be preferred (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Known CYP2D6 poor metabolisers_ In patients who are known to be CYP2D6 poor metabolisers: - One injection start: The starting dose should be 300 mg Abilify Maintena and continue treatment with prescribed dose of oral aripiprazole per day for 14 consecutive days. - Two injection start: The starting dose should be 2 separate injections of 300 mg Abilify Maintena (see method of administration) along with one single dose of the previous prescribed dose of oral aripiprazole. After the injection start, the maintenance dose of Abilify Maintena in known CYP2D6 poor metabolisers is 300mg. In patients who are known to be CYP2D6 poor metabolisers and concomitantly use a strong CYP3A4 inhibitor: - The one injection start: The starting dose should be reduced to 200 mg (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) and continue treatment with the prescribed dose of oral aripiprazole per day for 14 consecutive days. - Two injection start is not to be used in patients who are known to be CYP2D6 poor metabolisers and concomitantly use a strong CYP3A4 inhibitor. After the injection start, see table below for the recommended maintenance dose of Abilify Maintena. Abilify Maintena should be administered once monthly as a single injection (no sooner than 26 days after the previous injection). _Maintenance dose adjustments due to interactions with CYP2D6 and/or CYP3A4 inhibitors and/or CYP3A4 inducers_ Maintenance dosage adjustments should be done in patients taking concomitant strong CYP3A4 inhibitors or strong CYP2D6 inhibitors for more than 14 days. If the CYP3A4 inhibitor or CYP2D6 inhibitor is withdrawn, the dosage may need to be increased to the previous dose (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In case of adverse reactions despite dose adjustments of Abilify Maintena, the necessity of concomitant use of CYP2D6 or CYP3A4 inhibitor should be reassessed. Concomitant use of CYP3A4 inducers with Abilify Maintena should be avoided for more than 14 days because the blood levels of aripiprazole are decreased and may be below the effective levels (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Maintenance dose adjustments of Abilify Maintena in patients who are taking concomitant strong CYP2D6 inhibitors, strong CYP3A4 inhibitors, and/or CYP3A4 inducers for more than 14 days** 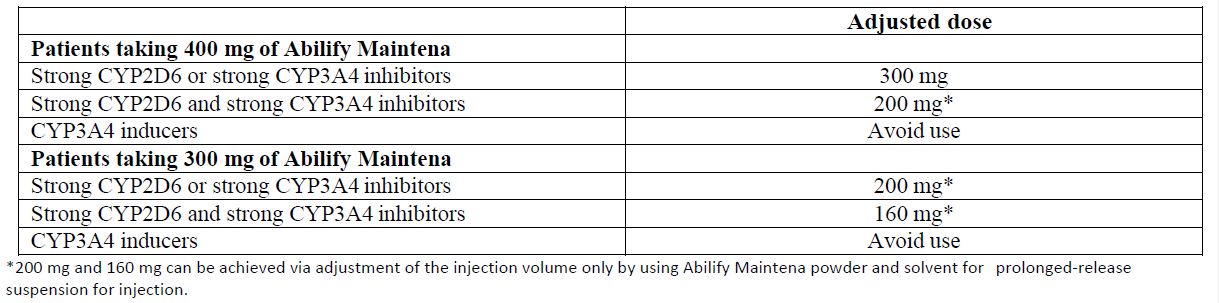 Paediatric population The safety and efficacy of Abilify Maintena in children and adolescents aged 0–17 years have not been established. No data are available. Method of administration Abilify Maintena is only intended for intramuscular use and should not be administered intravenously or subcutaneously. It should only be administered by a healthcare professional. The suspension should be injected immediately after reconstitution but can be stored below 25 °C for up to 4 hours in the vial. The suspension should be injected slowly as a single injection (doses must not be divided) into the gluteal or deltoid muscle. Care should be taken to avoid inadvertent injection into a blood vessel. Sites of injections should be rotated between the two gluteal or deltoid muscles. If initiating with the two injection start, inject into two different sites in two different muscles. DO NOT inject both injections concomitantly into the same deltoid or gluteal muscle. For known CYP2D6 poor metabolisers administer in either two separate deltoid muscles or one deltoid and one gluteal muscle. DO NOT inject into two gluteal muscles. The recommended needle for gluteal administration is a 38 mm (1.5 inch), 22 gauge hypodermic safety needle. For obese patients (Body mass index > 28 kg/m2), a 51 mm (2 inch), 21 gauge hypodermic safety needle should be used. The recommended needle for deltoid administration is a 25 mm (1 inch), 23 gauge hypodermic safety needle. For obese patients, a 38 mm (1.5 inch), 22 gauge hypodermic safety needle should be used (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The powder and solvent vials and the pre-filled syringe are for single-use only. For instructions on reconstitution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAMUSCULAR
Medical Information
**4.1 Therapeutic indications** - For the acute and maintenance treatment of schizophrenia in adults - For maintenance treatment to prevent the recurrence of manic or mixed episodes of bipolar I disorder in adult patients as monotherapy
**4.3 Contra-indications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
N05AX12
aripiprazole
Manufacturer Information
LUNDBECK SINGAPORE PTE. LTD.
Otsuka Pharmaceutical Co.Ltd
Siegfried Hameln GmbH (Water for Injection)
Otsuka Pharmaceutical Co., Ltd.
Active Ingredients
Sterile Aripiprazole monohydrate 416mg/vial equivalent to anhydrous Aripiprazole
400mg/vial
Documents
Package Inserts
Needle Guide Terumo SurGuard (IFU).pdf
Approved: August 16, 2017
