Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Treatment must be initiated and supervised by a physician experienced in the use of anticancer therapies. Prior to initiation of treatment with TEPMETKO the presence of _MET_ ex14 skipping alterations should be confirmed by a validated test method using nucleic acids isolated from plasma or tumour specimens. Posology The recommended dose is 450 mg tepotinib (2 tablets) taken once daily. Treatment should continue until disease progression or unacceptable toxicity. If a daily dose is missed, it can be taken as soon as remembered on the same day, unless the next dose is due within 8 hours. _Dose modification for adverse reactions_ If pulmonary symptoms indicative of interstitial lung disease (ILD)-like reactions occur, TEPMETKO should be withheld and patients should be promptly investigated for alternative diagnosis or specific aetiology of interstitial lung disease. TEPMETKO must be permanently discontinued if interstitial lung disease is confirmed and the patient treated appropriately (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended dose reduction of TEPMETKO for the management of adverse reactions is 225 mg orally once daily. Permanently discontinue TEPMETKO in patients who are unable to tolerate 225 mg orally once daily. The recommended dosage modifications of TEPMETKO for adverse reactions are provided in Table 1. 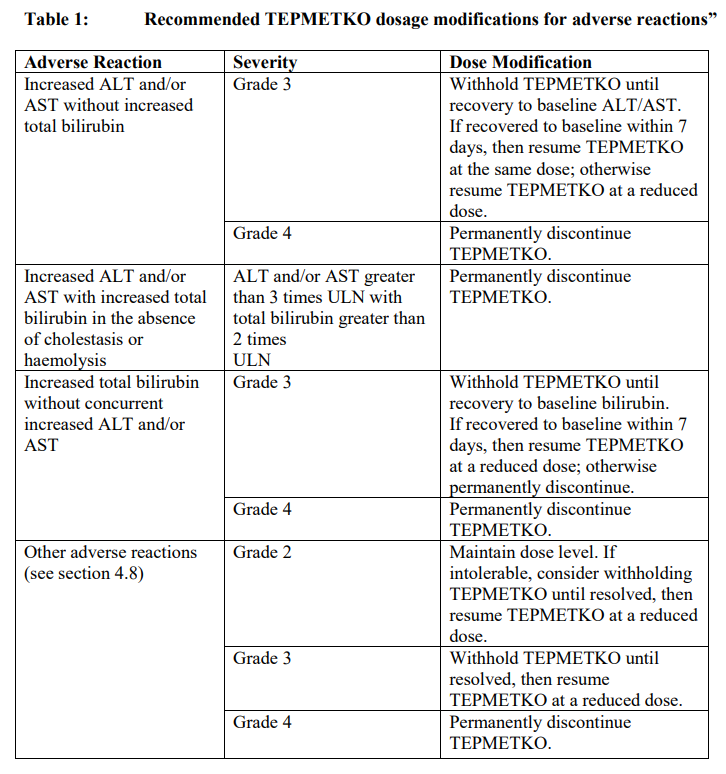 _Renal impairment_ No dose adjustment is recommended in patients with mild or moderate renal impairment (creatinine clearance 30 to 89 mL/min) (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The pharmacokinetics and safety of tepotinib in patients with severe renal impairment (creatinine clearance below 30 mL/min) have not been studied. _Hepatic impairment_ No dose adjustment is recommended in patients with mild (Child Pugh Class A) or moderate (Child Pugh Class B) hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The pharmacokinetics and safety of tepotinib in patients with severe hepatic impairment (Child Pugh Class C) have not been studied. _Elderly_ No dose adjustment is necessary in patients aged 65 years and above (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ Safety and effectiveness of TEPMETKO in paediatric patients below 18 years of age have not been established. Method of administration TEPMETKO is for oral use. The tablet(s) should be taken with food and should be swallowed whole. Administration to patients who have difficulty swallowing solids If the patient is unable to swallow, the tablets can be dispersed in 30 mL of non-carbonated water. No other liquids should be used or added. Drop the tablets in a glass with water without crushing, stir until the tablets dispersed into small pieces (the tablet will not completely dissolve) and swallow the dispersion immediately or within 1 hour. Do not chew pieces of the tablet. Rinse with additional 30 mL and drink immediately to ensure that no residues remain in the glass and the full dose is administered. If an administration via a naso-gastric tube (with at least 8 French gauge) is required, disperse the tablets in 30 mL of non-carbonated water as described above. Administer the 30 mL of liquid immediately or within 1 hour as per naso-gastric tube manufacturer’s instructions. Immediately rinse twice with 30 mL each to ensure that no residues remain in the glass or syringe and the full dose is administered.
ORAL
Medical Information
**4.1 Therapeutic indications** TEPMETKO is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) harbouring mesenchymal-epithelial transition factor gene ( _MET_) exon 14 ( _MET_ ex14) skipping alterations.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01EX21
tepotinib
Manufacturer Information
MERCK PTE. LTD.
Merck Healthcare KGaA
Active Ingredients
Documents
Package Inserts
Tepmetko film coated tablet PI.pdf
Approved: April 13, 2023
